Lecture 21 BRACHYTHERAPY Lecture 21 Ahmed Group
39 Slides7.21 MB

Lecture 21 BRACHYTHERAPY Lecture 21 Ahmed Group

Brachytherapy -is the internal radiation treatment achieved by implanting radioactive material directly into the tumor or very close to it. Sometimes called internal radiation therapy. Prefix “brachy” – from Greek for “short range” Implanting radioactive sources directly into a tumor was a strategy first suggested by Alexander Graham Bell soon after the turn of the century. Lecture 21 Ahmed Group

Brachytherapy There are two distinct forms of brachytherapy: 1) Intracavitary irradiation using radioactive sources that are placed in body cavities in close proximity to the tumor and 2) Interstitial brachytherapy using radioactive seeds implanted directly into the tumor volume. Lecture 21 Ahmed Group

Lecture 21 Dose rate effects (HDR and LDR) Choice of isotopes Interstitial and intra-cavitary use Radio-labeled antibodies BED and Iso-effective dose calculations Ahmed Group

Low Dose Intracavitary Brachytherapy Low dose intracavitary brachytherapy (dose rate of about 50cGy/hr, or 50 rad/h) is temporary and usually takes 1 to 4 days. It is most commonly used for the uterine cervix. Lecture 21 Ahmed Group

High Dose Intracavitary Brachytherapy To an increasing extent, low-dose-rate intracavitary brachytherapy is being replaced by high-dose-rate intracavitary therapy, delivered in 3 to 12 dose fractions. This therapy gives up much of the radiobiological advantage and the sparing of late-responding normal tissues. It is only possible because the treatment of carcinoma of the cervix is a special case in which the dose-limiting normal tissues (e.g. bladder, rectum) receive a lower dose than the dose to the tumor. Lecture 21 Ahmed Group

High Dose Intracavitary Brachytherapy For high-dose-rate treatments lasting a few minutes, it is possible to sue retractors that result in even lower doses to the critical normal tissues that are possible with an insertion that lasts 24 hours or more. These physical advantages offset the radiobiologic disadvantages, so that the general principle, that administration of a few large fractions at a high dose rate gives poorer results than a lower dose rate, dose not apply in this case. Lecture 21 Ahmed Group

Lecture 21 Dose rate effects (HDR and LDR) Choice of isotopes Interstitial and intra-cavitary use Radio-labeled antibodies BED and Iso-effective dose calculations Ahmed Group
Choice of isotopes There has been a continual evolution in the radionuclide used; in the early days, radium was used, but this went out of favor because of the safety concern of using an encapsulated source that can leak radioactivity. As an interim measure, cesium-137 was introduced, but today most treatment centers use iridium-192; its shorter half-life and lower gamma-ray energy make for ease of radiation protection, especially in conjunction with a remote after loader. Lecture 21 Ahmed Group

Lecture 21 Dose rate effects (HDR and LDR) Choice of isotopes Interstitial and intra-cavitary use Radio-labeled antibodies BED and Iso-effective dose calculations Ahmed Group

Interstitial Brachytherapy Interstitial brachytherapy permanent. can be either temporary or Temporary brachytherapy. Most widely used radionuclide at the present time is iridium192 Lecture 21 Ahmed Group
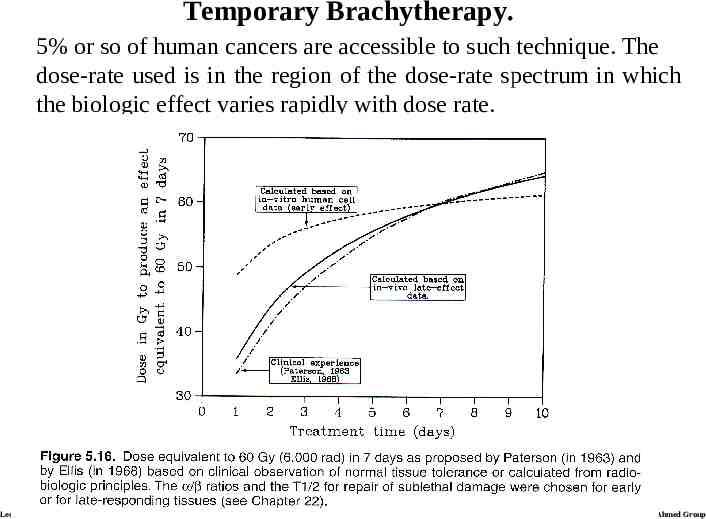
Temporary Brachytherapy. 5% or so of human cancers are accessible to such technique. The dose-rate used is in the region of the dose-rate spectrum in which the biologic effect varies rapidly with dose rate. Lecture 21 Ahmed Group
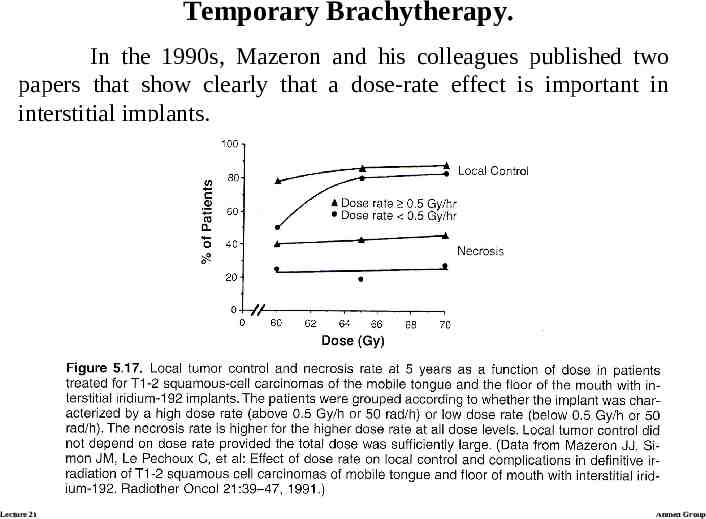
Temporary Brachytherapy. In the 1990s, Mazeron and his colleagues published two papers that show clearly that a dose-rate effect is important in interstitial implants. Lecture 21 Ahmed Group
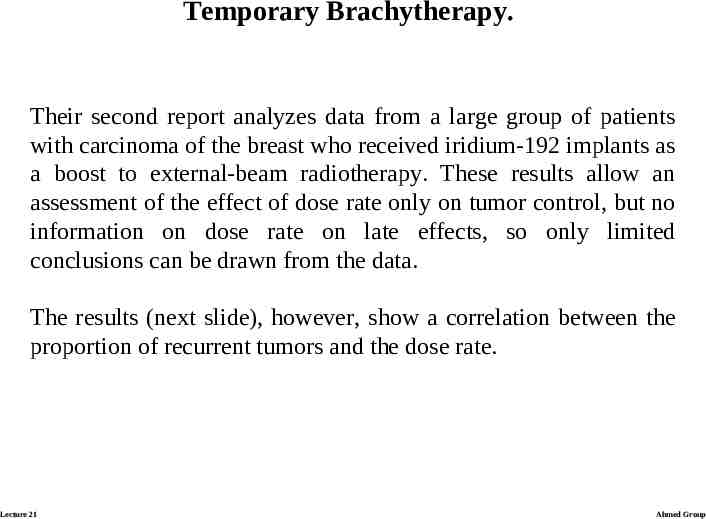
Temporary Brachytherapy. Their second report analyzes data from a large group of patients with carcinoma of the breast who received iridium-192 implants as a boost to external-beam radiotherapy. These results allow an assessment of the effect of dose rate only on tumor control, but no information on dose rate on late effects, so only limited conclusions can be drawn from the data. The results (next slide), however, show a correlation between the proportion of recurrent tumors and the dose rate. Lecture 21 Ahmed Group
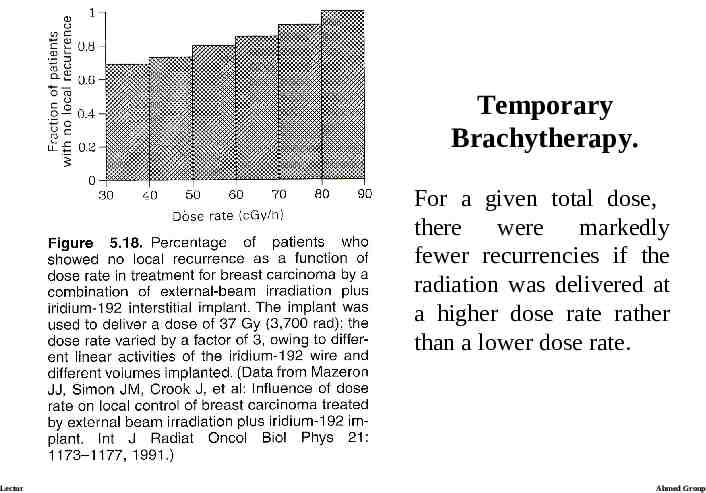
Temporary Brachytherapy. For a given total dose, there were markedly fewer recurrencies if the radiation was delivered at a higher dose rate rather than a lower dose rate. Lecture 21 Ahmed Group

Temporary Brachytherapy. The relatively short half-life of iridium-192 (70 days) means that a range of dose rates is inevitable. It is important, therefore, to correct the total dose rate. Iridium-192 has two advantages: 1) The source size can be small, and 2) Its lower photon energy makes radiation protection easier than with radium or cesium-137 Lecture 21 Ahmed Group
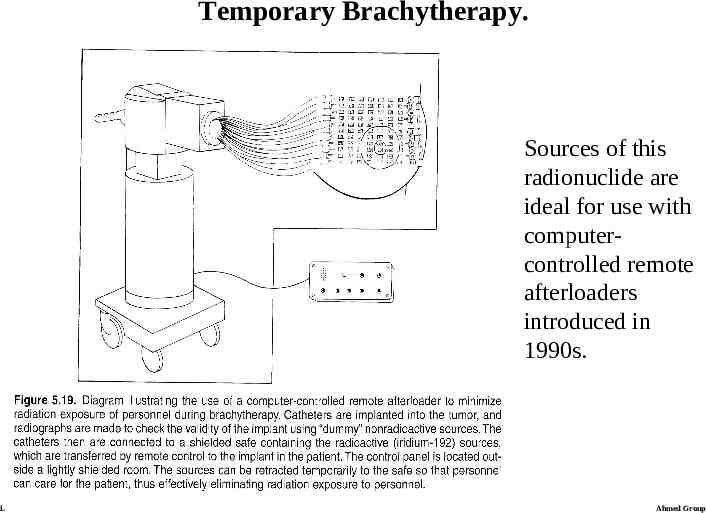
Temporary Brachytherapy. Sources of this radionuclide are ideal for use with computercontrolled remote afterloaders introduced in 1990s. Lecture 21 Ahmed Group

Permanent Interstitial Implants Encapsulated sources with relatively short half-life can be left in place permanently. There are two advantages for the patient: 1) An operation to remove the implant is not needed, 2) the patient can go home with the implant in place. Iodine-125 has been used most widely to date for permanent implants. The total prescribed dose is usually about 160 Gy at the periphery of the implanted volume, with 80 Gy delivered in the first half-life of 60 days. A major advantage of iodine-125 is the low energy of the photons emitted (about 30 keV). Lecture 21 Ahmed Group
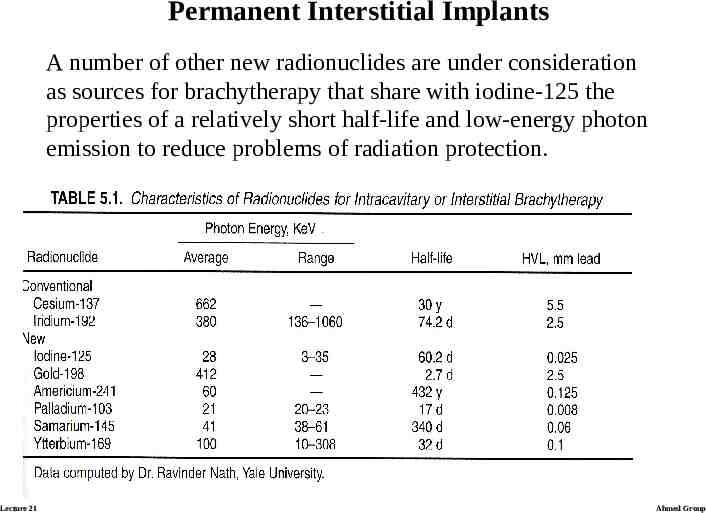
Permanent Interstitial Implants A number of other new radionuclides are under consideration as sources for brachytherapy that share with iodine-125 the properties of a relatively short half-life and low-energy photon emission to reduce problems of radiation protection. Lecture 21 Ahmed Group

Lecture 21 Dose rate effects (HDR and LDR) Choice of isotopes Interstitial and intra-cavitary use Radio-labeled antibodies BED and Iso-effective dose calculations Ahmed Group
Radio-labeled antibodies Radio-labeled immunoglobulin therapy is radiotherapy for cancer using an antibody to deliver a radioactive isotope to the tumor. Much of the pioneering work in this field was done by Stanley Order and his colleagues in the 1980s, with the primary focus on anti-ferritin labeled with radioactive iodine or yttrium. Although ferritin is also present in normal tissues, selective tumor targeting has been demonstrated in animal models and in clinical scanning, historically performed first for Hodgkin’s disease. This differential is the basis of the potential therapeutic gain in radio-labeled immunoglobulin therapy. Radio-labeled murine monoclonal antibodies (in oppose to earlier used polyclonal antibodies) carrying iodine-131, are being currently used for both diagnosis and therapy. More recently, chimeric mouse-human antibodies have become available for radiotherapy Lecture 21 Ahmed Group

Radiolabeled antibodies In the last 20 years new tumor markers have been described as a result of developments in the field of protein chemistry and hybridoma technology. Antibodies to tumor-related products are regularly used to assist the histological and serological diagnosis of many types of cancer and monitor therapy. A further and very exciting role of antibodies is to act as vehicles to target therapy to tumors. The possibility of investigating the use of these antibodies to target tumors has been greatly assisted by techniques such as human xenografts in animals. Lecture 21 Ahmed Group
Radiolabeled antibodies There are two general strategies that can be employed for therapeutic antibody targeting. One is serotherapy which uses the host’s immune system to interact with the anti-tumor antibody to kill tumor cells. In the other and more frequently used approach a cytocidal agent is conjugated to an antibody which carries the compound to the tumor. Lecture 21 Ahmed Group

Radionuclide conjugates The selection of a particular radionuclide depends on tumor size. High energy beta-radiation is best suited to larger tumors where neighbouring areas may benefit from cross-fire effects. However, it is not clear whether the different characteristics of radionuclides are sufficiently important to produce significant therapeutic differences in the results of radio-immunotherapy. Despite potential radiobiological advantages there are practical considerations that influence the choice of radionuclide. Lecture 21 Ahmed Group
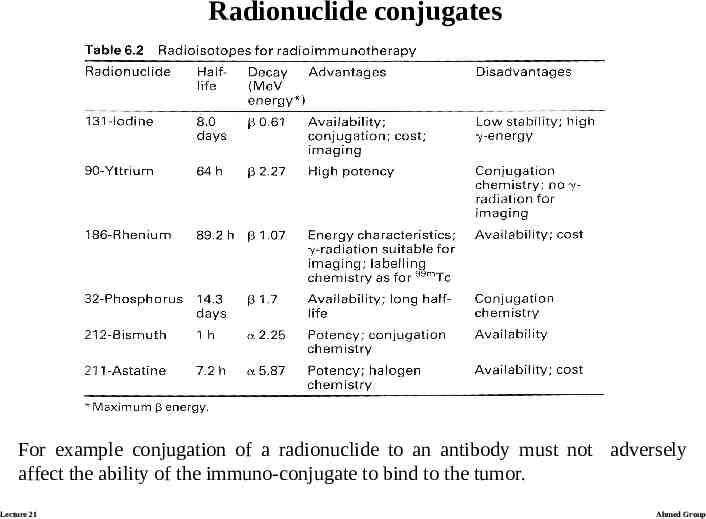
Radionuclide conjugates For example conjugation of a radionuclide to an antibody must not adversely affect the ability of the immuno-conjugate to bind to the tumor. Lecture 21 Ahmed Group
Clinical trials with radio-labeled antibodies Radio-labeled antibodies have now been used to treat many types of cancer. Therapeutic studies with radio-labeled antibodies: Intravenous: Hepatoma Lymphoma Colorectal cancer Melanoma Neuroblastoma Intraperitoneal/Intrapleural Ovarian cancer Lung cancer Intra-thecal Neuroblastoma Malignant meningitis Intra-arterial Glioma Lecture 21 Ahmed Group
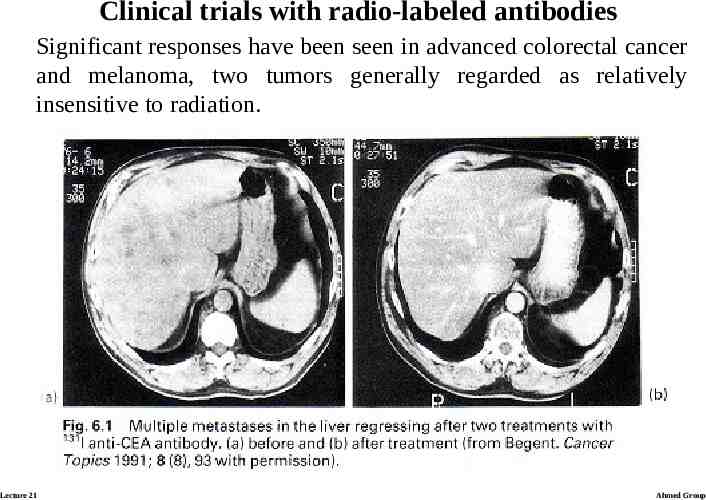
Clinical trials with radio-labeled antibodies Significant responses have been seen in advanced colorectal cancer and melanoma, two tumors generally regarded as relatively insensitive to radiation. Lecture 21 Ahmed Group
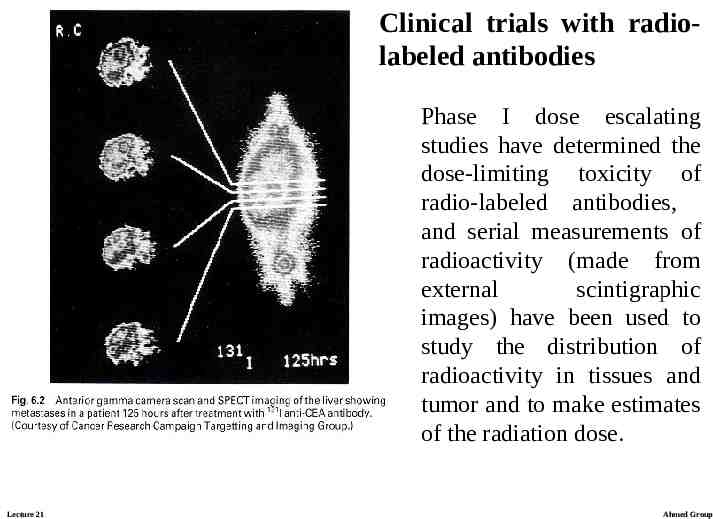
Clinical trials with radiolabeled antibodies Phase I dose escalating studies have determined the dose-limiting toxicity of radio-labeled antibodies, and serial measurements of radioactivity (made from external scintigraphic images) have been used to study the distribution of radioactivity in tissues and tumor and to make estimates of the radiation dose. Lecture 21 Ahmed Group
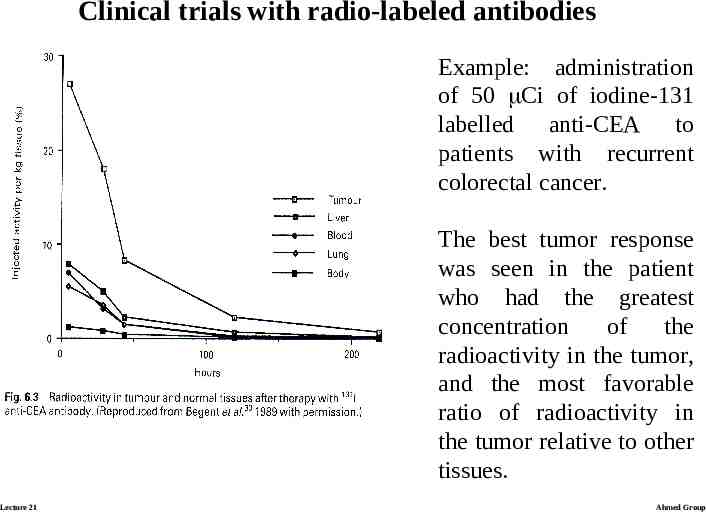
Clinical trials with radio-labeled antibodies Example: administration of 50 Ci of iodine-131 labelled anti-CEA to patients with recurrent colorectal cancer. The best tumor response was seen in the patient who had the greatest concentration of the radioactivity in the tumor, and the most favorable ratio of radioactivity in the tumor relative to other tissues. Lecture 21 Ahmed Group
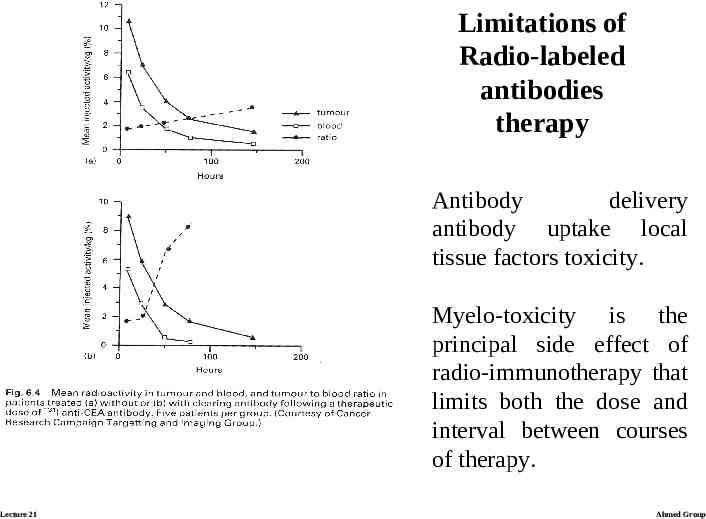
Limitations of Radio-labeled antibodies therapy Antibody delivery antibody uptake local tissue factors toxicity. Myelo-toxicity is the principal side effect of radio-immunotherapy that limits both the dose and interval between courses of therapy. Lecture 21 Ahmed Group

Lecture 21 Dose rate effects (HDR and LDR) Choice of isotopes Interstitial and intra-cavitary use Radio-labeled antibodies BED and Iso-effective dose calculations Ahmed Group
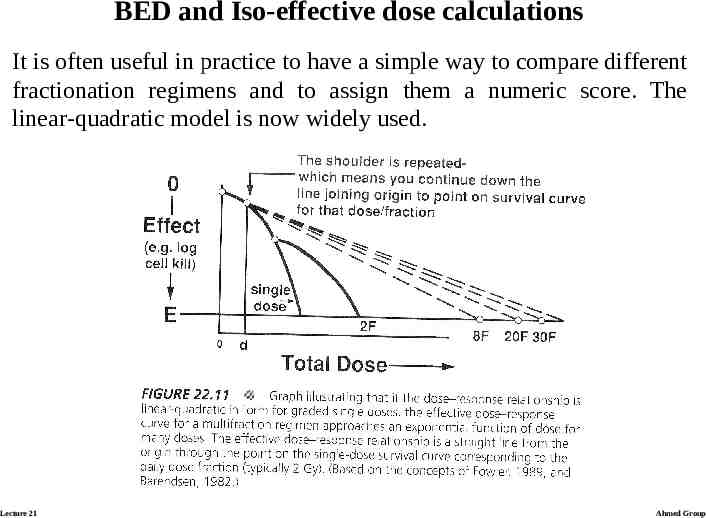
BED and Iso-effective dose calculations It is often useful in practice to have a simple way to compare different fractionation regimens and to assign them a numeric score. The linear-quadratic model is now widely used. Lecture 21 Ahmed Group
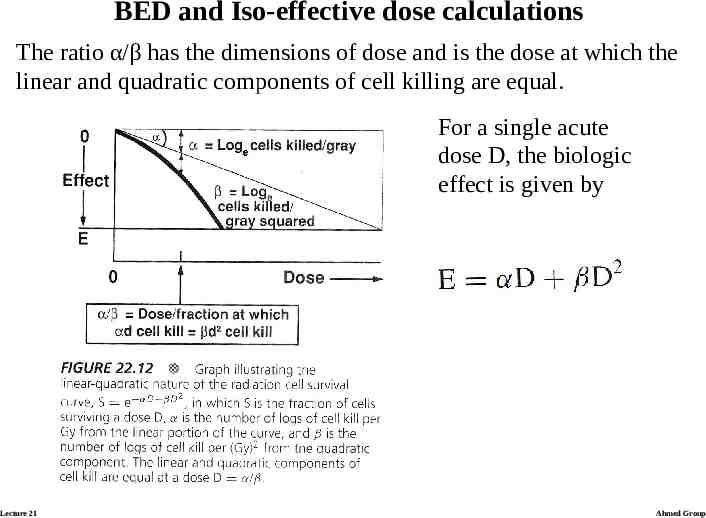
BED and Iso-effective dose calculations The ratio α/β has the dimensions of dose and is the dose at which the linear and quadratic components of cell killing are equal. For a single acute dose D, the biologic effect is given by Lecture 21 Ahmed Group
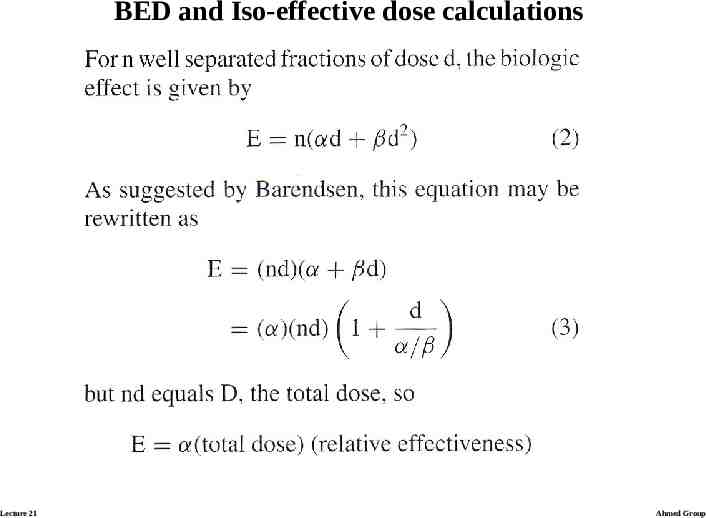
BED and Iso-effective dose calculations Lecture 21 Ahmed Group
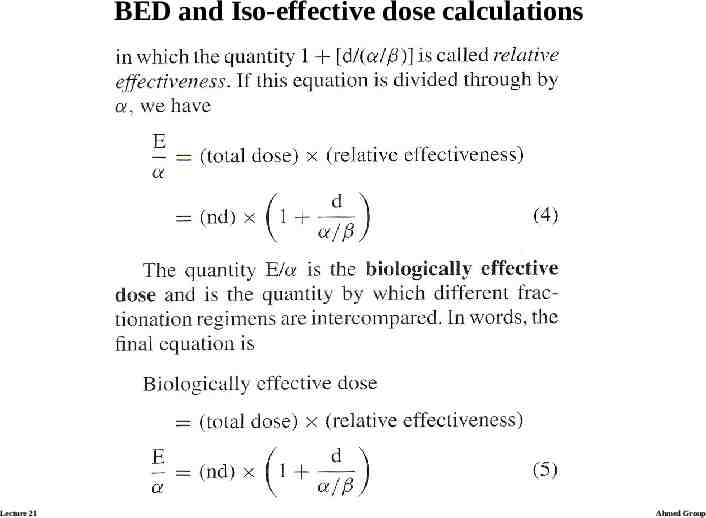
BED and Iso-effective dose calculations Lecture 21 Ahmed Group
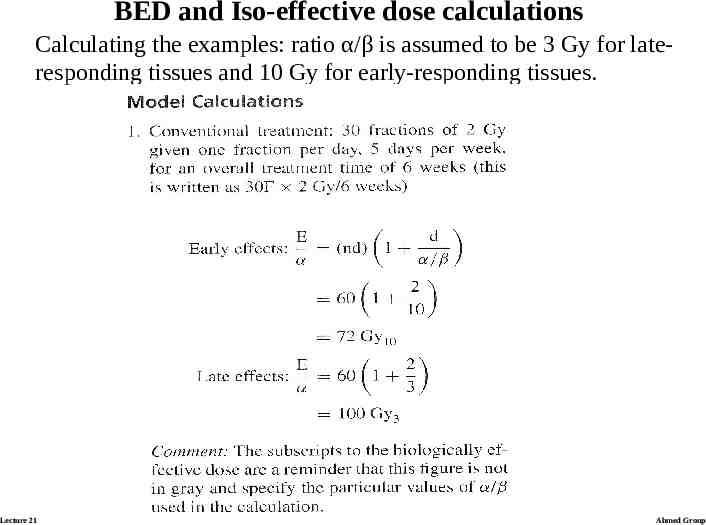
BED and Iso-effective dose calculations Calculating the examples: ratio α/β is assumed to be 3 Gy for lateresponding tissues and 10 Gy for early-responding tissues. Lecture 21 Ahmed Group
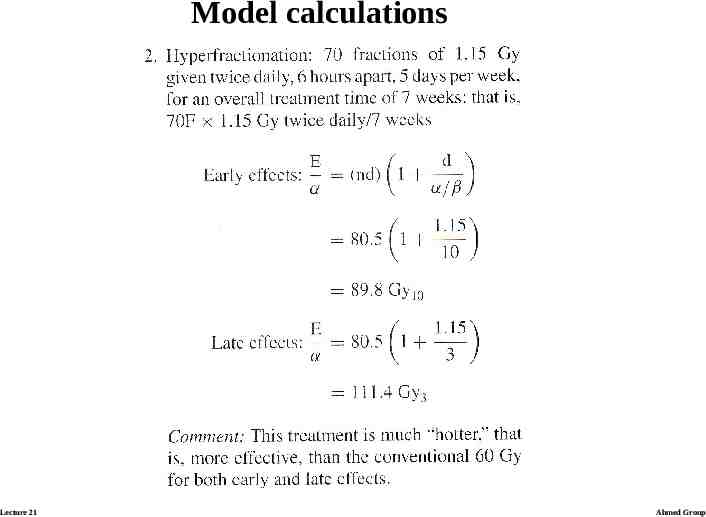
Model calculations Lecture 21 Ahmed Group
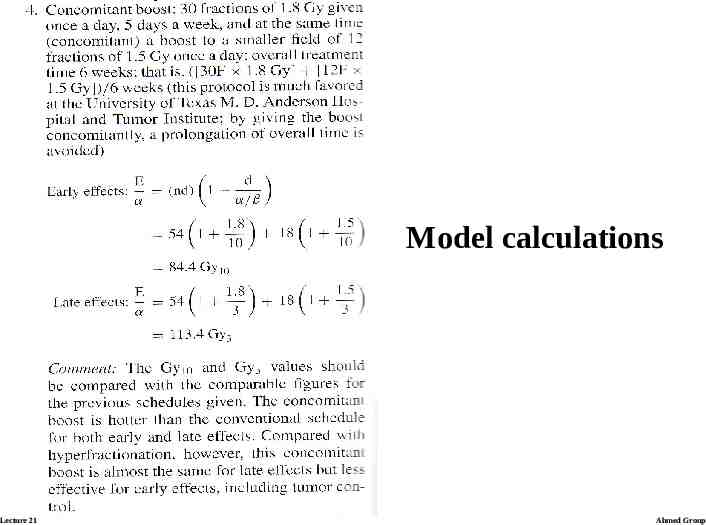
Model calculations Lecture 21 Ahmed Group
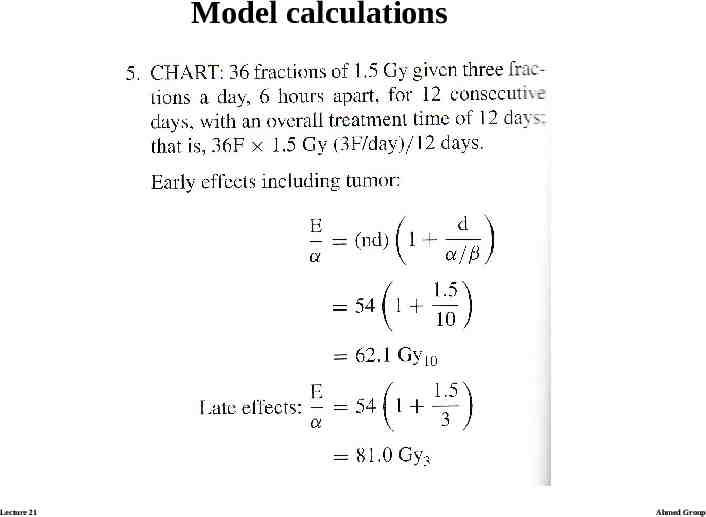
Model calculations Lecture 21 Ahmed Group






