PQRI Survey of Pharmaceutical Excipient Testing and Control
29 Slides135.00 KB

PQRI Survey of Pharmaceutical Excipient Testing and Control Strategies Used by Excipient Manufacturers, Excipient Distributors and Drug-Product Manufacturers 1 PQRI Opening Session

Welcome When? The time is NOW! Where? The place is HERE! 2 The next 2 days provide the opportunity for you to participate! Please be respectful of time limits. This hotel wing will break down to 5 discussion areas. Your individualized, randomized topic sequence ensures you will discuss each topic. See your personal packet. You will hear the thoughts of all other participants at least once. PQRI Opening Session

Support 3 The Workshop Booklet PQRI assistance – Sylvia Gantt Hotel maps Phones Facilities PQRI Opening Session

PQRI Working Group Members Pharmaceutical Quality Research Institute's Excipient Working Group has organized the survey and workshop. 4 Gregory Larner, Statistics Manager with Pfizer (Kalamazoo, MI) David Schoneker, Chair-elect, IPEC-Americas and Director of Global Regulatory Affairs at Colorcon (West Point, PA). Catherine Sheehan, Director for Excipients Group at USP, (Rockville, MD) Rajendra Uppoor, Pharmacist, Office of Pharmaceutical Science, CDER/FDA (Silver Spring, MD). Phyllis Walsh, Senior Compendial Manager with Schering-Plough (Kenilworth, NJ). Robert Wiens, Compendial Affairs/Global Method Management, Eli Lilly (Indianapolis, IN). PQRI Opening Session

The Workshop PQRI Excipient Working Group welcomes you to a Workshop on Excipient Control Strategies. Safe and Effective Excipients is our Goal 5 Component Regulations and Control is critical Global economy increases complexity Need effective and efficient control strategies PQRI Opening Session

Objectives – PQRI Working Group 6 Provide a report on the Survey of Excipient Testing and Control Strategies Determine the impact of those findings on stakeholders. Hold discussions on the impact of FDA regulation and guidance on excipient control strategies and how to use them correctly and effectively. PQRI Opening Session

Objectives - Participants Based on the survey information and conference discussions the participants will: 7 Identify concerns of stakeholders - including excipient manufacturers, drug product manufacturers, regulators, and USP. Communicate and clarify regulations. Develop stakeholder recommendations and ideas for potential changes in compendia, guidances and regulations to minimize regulatory burden. PQRI Opening Session

Deliverables 8 Summaries of workshop discussions A Joint Position Paper from excipient manufacturers, drug-product manufacturers and USP on key excipient issues. Possible solutions to issues currently faced by the stakeholders. PQRI Opening Session

How the Survey was Conducted PQRI conducted an open, publicly available, electronic survey Current excipient-control strategies were studied. 3 surveys were available: 9 pharmaceutical excipient manufacturers, excipient distributors and drug-product manufacturers (excipient users) PQRI Opening Session

Survey Highlights - I 10 Nearly all respondents (99%) stated that their excipient specifications comply with USP-NF monograph requirements. Almost all drug product manufacturers (97%) test excipients according to USP-NF monograph/general chapter methods; Approximately 1 in 6 excipient manufacturers and excipient distributors do not test according to USP-NF. PQRI Opening Session

Survey Highlights - II 11 Most (79%) respondents (excipient manufacturers, excipient distributors, and excipient users) have been inspected by the Food and Drug Administration (FDA); and most distributors have been inspected by their State or Local Authorities. Most excipient specifications are both national (USP-NF) and global, versus up to 15% just national (USP-NF). PQRI Opening Session

Survey Highlights - III Most excipients obtained from new vendor sources are qualified An excipient from a new supplier (or vendor), is qualified 12 by vendor audit (91%) and complete testing according to compendial monograph (96%) for the article. approximately 35% of the time by supplier’s analytical method, about 50% by an in-house method, 63% of the time by process validation in the dosage form, but rarely (15%) accepted on Certificate of Analysis (C of A) with identity test alone. PQRI Opening Session
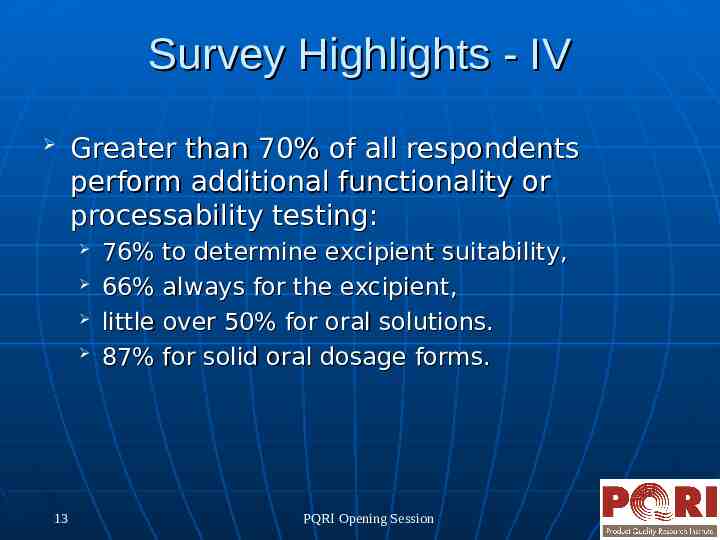
Survey Highlights - IV Greater than 70% of all respondents perform additional functionality or processability testing: 13 76% to determine excipient suitability, 66% always for the excipient, little over 50% for oral solutions. 87% for solid oral dosage forms. PQRI Opening Session

Survey Highlights - V 85% of drug product manufacturers, and all distributors have a vendor certification program. 87% of drug product manufacturers audit 14 excipient manufacturing sites, and testing sites. Greater than 90% of drug product manufacturers’ audits are done “on-site of the vendor” by their own company auditors less than 20% by third party, 53% of the audits include a questionnaire. PQRI Opening Session

Background 15 2003 – European Agency for the Evaluation of Medicinal Products issued guidance for excipients and US Food and Drug Administration issued guidance for CMC. Industry believed that generally accepted excipient control strategies were eliminated by the guidance. PQRI Opening Session

Background, continued Control strategies of concern 16 Drug Product Manufacturer (DPM) may apply internal method, equivalent to Pharmacopeia. Select single method capable of ensuring compliance with multiple Pharmacopeia. 2006 – FDA strategically focuses on CGMPs for 21st Century, including an announcement that the 2003 guidance is withdrawn. PQRI Opening Session

Surprises About 25% of the time drug product manufacturers test excipient suitability for processing, using experimental (laboratory) scale batches, or pilot scale manufacturing batches. Batches were rarely (15%) accepted on CofA with identity test alone. 17 This was higher than expected. This is an accepted approach in the CFR, and was lower than expected. PQRI Opening Session

Surprises, continued Most excipient manufacturers and distributors that replied to the survey do label their excipients as compendial grade. Excipient GMP requirements perceived as being too restrictive generally do not impact their decision; Low demand for compendial grade generally does not impact their decision; “Can not meet the compendial monograph” criteria generally does not impact their decision. 18 This may not be reflective of the entire excipient manufacturers industry since the pharmaceutical industry is often a small fraction of their business. PQRI Opening Session

Summary of Key Survey Findings The majority of excipient manufacturers; excipient distributors and drug product manufacturers 19 Manufacture their products for global distribution. Test their excipients according to USP-NF monograph and general chapter methods. 97% of drug product manufacturers perform more than an identification test when receiving excipients from their vendors along with Certificate of Analysis. PQRI Opening Session

Summary of Key Survey Findings New sources of excipients used by drug product manufacturers are qualified by Nearly half (40%) of drug product manufacturers had difficulty in finding a manufacturer of USP-NF grade excipient. 20 vendor audits, and complete compendial testing. In such a situation, they would use the best grade available, test the excipient according to compendial monograph and conduct the excipient manufacturer’s assessment. PQRI Opening Session

Summary of Key Survey Findings 75% of drug product manufacturers indicated they ensure few to all excipients they use conform to compendial grade by testing, along with manufacturer’s site audits. In 80% of the cases, validated test procedures are used to show: 21 a noncompendial grade excipient conforms to a compendial grade, or a compendial grade conforms to a multi-compendia grade. PQRI Opening Session

Summary of Key Survey Findings Majority of excipient manufacturers and distributors are not concerned about such factors as: 22 Excipient GMP requirements being restrictive or low demand for compendial grade, or inability to meet compendial monograph requirement, or potential to be inspected by FDA, or audits by drug product manufacturers. Nearly 80% of excipient manufacturers, distributors and drug product manufacturers have been inspected or visited by the FDA or State or local authorities. PQRI Opening Session

Summary of Key Survey Findings 89% of drug product manufacturers stated that at least 5 of their excipients are in a reduced testing program. They do not perform complete monograph testing after vendor qualification and receipt of Certificate of Analysis. Excipient manufacturers, distributors and drug product manufacturers responded to be adequately familiar with: 23 FDA and compendial requirements and recommendations for excipients testing. PQRI Opening Session

Summary of Key Survey Findings 70% excipient manufacturers, distributors, and drug product manufacturers perform additional functionality or processability testing that is not part of any compendial monograph: 24 87% due to processing concerns 87% for solid oral dosage forms 24% of drug product manufacturers have products for which excipient variability is a problem in spite of such extra-compendial testing. PQRI Opening Session

Summary of Key Survey Findings Alternate international compendial methods are used by half or more of excipient manufacturers, distributors and drug product manufacturers to test some, most or all of their excipients instead of USP-NF. Nearly 60% of excipient manufacturers and drug product manufacturers 25 conduct excipient testing per harmonized monographs; and reduce redundant testing by demonstrating multiple compendial specification equivalence, or by using the most stringent method or specification. PQRI Opening Session

Summary of Key Survey Findings 26 About 50% of excipient manufacturers and drug product manufacturers have applied harmonized excipient monographs and harmonized general chapters across all their sites. PQRI Opening Session

Workshop Topics A and B A. B. 27 Clarify “continuous-flow manufacturing” and “skip-lot testing” used for excipients in the context of 21 CFR Part 211.84 regulations. Discuss how characterization of excipient physical and chemical properties helps build quality into the drug product. PQRI Opening Session

Workshop Topics C, D and E C. D. E. 28 Highlight advantages of increased use of third-party audits. Discuss strategies to increase the number of excipients labeled USP— NF. Discuss when reduced testing is appropriate. PQRI Opening Session

Round Table Discussions 29 Attendees will rotate through each topic. The topics will stay in the same room. Attendees will be randomized so you will meet all attendees and attend all topics. PQRI Opening Session