Comprehensiv e Cardiometabo lic RiskPhase 2 2009Reduction
51 Slides4.06 MB

Comprehensiv e Cardiometabo lic RiskPhase 2 2009Reduction Program Sponsored by National Lipid Association

Case Study Special Considerations for the Overweight/Obese Patient

Case Study Overview A 46-year-old male lawyer is referred by his physician for persistent weight gain and high cardiomyopathy risk Patient has hyperlipidemia and hypertension; comorbidities include asthma, attention-deficit/hyperactivity disorder (ADHD), chronic fatigue, and depression Family history of obesity, type 1 and 2 diabetes Current weight of 305.7 pounds is his highest – Admits poor nutritional habits and a low activity level Reports waking up “snorting” from snoring at night – Experiences morning headaches and daytime somnolence

The Regulation of Food Intake Is a Complex Process Brain Central Signals Stimulate NPY AGRP Galanin Orexin-A Dynorphin Peripheral Signals Glucose – CCK, GLP-1, Apo A-IV Vagal afferents Insulin Ghrelin – Inhibit -MSH CRH/UCN GLP-I CART NE 5-HT External Factors Emotions Food characteristics Lifestyle behaviors Environmental cues Peripheral Organs Gastrointestinal tract Adipose tissue Leptin Cortisol Adrenal glands Zhang Y, et al. Nature. 1994;372:425-432; Schwartz MW, et al. Nature. 2000;404:661-671. Food Intake NPY neuropeptide Y, AGRP agouti-related protein, α-MSH alpha-melanocyte-stimulating hormone, CRH/UCN corticotropin-releasing hormone/urocortin, GLP-1 glucagon-like peptide-1, CART cocaine- and amphetamineregulated transcript, NE norepinephrine, 5-HT seratonin, CCK cholecystokinin, Apo A-IV apolipoprotein A-IV.

Case Study Overview Medications – – – – – – Metoprolol 100-mg BID Atorvastatin 10-mg QD Niacin 1500-mg BID Paroxetine 40-mg QD Lithium 900-mg QD Amphetamine/ dextroamphetamine 40-mg QD

Starting Your Investigation Look for – – – – Obstructive sleep apnea (OSA) Medications causing weight-gain Depression Metabolic syndrome, prediabetes The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults . October 2000. NIH Publication No. 00-4084.

Clinical Pearl A Vicious Cycle Weight Gain Depression Sleep Apnea

Drug-Associated Weight-Change Reference Remember to keep this list in your office! 2007 Cardiometabolic Support Network

Case Study Medications That May Be Contributing to This Patient’s Excess Body Weight MAOIs monoamine oxidase inhibitors, TCAs tricyclic antidepressants, ACE angiotensin-converting enzyme

Case Study Laboratory Results Glucose: 106 mg/dL hs-CRP: 8.2 mg/L high risk TC: 184 mg/dL A1c: 5.9% HDL-C: 33 mg/dL Creatinine: 1.2 mg/dL LDL-C: 103 mg/dL AST: 27 U/L TG: 240 mg/dL ALT: 43 U/L eGFR: 60 mL/min Non–HDL-C: 151 mg/dL EKG: sinus bradycardia, rate 56 TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglycerides, AST aspartate aminotransferase, ALT alanine aminotranferase, hs-CRP high-sensitivity C-reactive protein

Case Study Initial Registered-Dietitian Appointment Weight: 305.1 pounds, height: 72 inches, BMI: 41.4 kg/m2, waist: 48 inches Former athlete with low activity level Volume eater with little sense of satiety “when he gets started” Daytime fatigue noted, being treated for ADHD Diet – Little fast food/red meat – Eats before bed and sometimes wakes up in the middle of the night to eat – Breakfast: nothing, lunch: salad, snack: fruit, dinner: Greek salad with chicken or stirfry, snack: “bad” Plan – Keep food records – Begin to eat breakfast – Eat higher lean-protein lunches and dinners and begin to reduce refined carbohydrates – Goal of 30 minutes of walking/day ADHD attention-deficit/hyperactivity disorder, BMI body mass index

Clinical Pearl High-frequency telephone- and web-based nutritional counseling can be effective ways to help patients lose weight

Clinical Pearl Breakfast and Nighttime Eating Skipping breakfast can drive nighttime eating – – – – Breakfast none Lunch breakfast Dinner lunch Nighttime snack dinner Nighttime eating drives skipping breakfast The cycle continues

ARS Question Which may be the best diet for someone with a lack of satiety? A. Low protein B. Low fat C. Low glycemic

Glycemic Index (GI) Although data vary, a low-GI meal may reduce subsequent energy intake1 Cochrane systematic review indicates that decreasing the GI* of a diet may be an effective way to promote weight-loss and improve lipid profiles2 *GI area under the curve (AUC) of the 2-hour blood glucose response curve divided by the AUC of an equal amount of glucose, multiplied by 100 Low GI food/meal 55 or less 1. Flint A, et al. Am J Clin Nutr. 2006;84:1365-73. 2. Thomas DE, et al. Cochrane Database Syst Rev. 2009;(1):CD005105.pub2.

Clinical Pearl Diet: What Is Most Important? Calorie restriction, with high macronutrient quality* *High quality indicates more than 5 servings of fruits and vegetables/day, lean protein sources including some vegetarian sources, nuts, healthy oils, nonfat dairy products, whole grains, low in sweets and refined carbohydrates, low in fat

Favorable Option for This Patient Low refined-carbohydrate diet with increased fiber intake – – – – Patient has prediabetes Rapid weight-loss is desirable Patient’s snacks tend to be refined carbohydrates Lower refined-carbohydrates reduce hunger in some patients – Higher fiber associated with satiety Higher protein intake – Protein increases satiety – Lean protein has little fat and saturated fat, making it a healthy option for weight loss

Practical Tips: Increasing Fiber and Lean Protein Fiber – Fiber One bran cereal Sprinkle it on low-fat yogurt as a bedtime snack – Whole grains, fruits, and vegetables Lean protein – Ham, turkey, and roast beef are the leanest sandwich meats Have 1/2 sandwich, but double the thickness – Carnation Instant Breakfast No Sugar Added with skim milk inexpensive, low-GI meal replacement – Modified pastas that are no longer “refined carbohydrates” Barilla PLUS (2-cups cooked) 17-g protein, 7-g fiber, 360mg omega-3 fatty acid

Case Study Initial MD Appointment Weight: 305.7 lbs, height: 72 inches, BMI: 41.5 kg/m2, blood pressure: 138/90, heart rate: 68 bpm, waist: 48 inches Patient is at his highest weight – Several prior weight-loss attempts: no significant progress, has been steadily gaining weight – Admits poor nutritional habits and a low activity level Reports waking up “snorting” from snoring at night – Has morning headaches and daytime somnolence Food records show nighttime eating pattern, with large quantities consumed after 6:00 PM

Case Study Medications – – – – – – Metoprolol 100-mg BID Atorvastatin 10-mg QD Niacin 1500-mg BID Paroxetine 40-mg QD Lithium 900-mg QD Amphetamine/dextroamphetamine 40-mg QD Action plan – – – – Reinforce importance of continued dietitian visits Sleep study to evaluate for obstructive sleep apnea Stop metoprolol; initiate ramipril, titrate to 5-mg BID Begin metformin ER 500-mg QD, with goal to increase

Clinical Pearl What if β-Blockers Are Necessary? If a β-blocker is necessary as part of a multi-agent antihypertensive regimen, an agent that does not aggravate insulin resistance (eg, carvedilol) may be a favorable choice

ARS Question According to the 2007 ADA Consensus Statement on impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), which of the following is not true? Metformin is appropriate for use in patients with IFG, IGT, and A. A1c 5.0% B. Hypertension C. BMI 35 kg/m2 D. Family history of diabetes in first-degree relative ADA American Diabetes Association, BMI body mass index

Pharmacological Intervention in the Progression to Diabetes: Recent Statements ADA 2007 Consensus Statement – Metformin as an adjunct/alternative to lifestyle in patients with IFG and IGT, and any of the following 60 years of age, BMI 35 kg/m2, family history of type 2 diabetes in first-degree relative, triglycerides, HDL-C, hypertension, A1C 6.0% ACE 2008 Consensus Statement – Metformin or acarbose as an adjunct to lifestyle in patients with prediabetes at particularly high risk ADA American Diabetes Association, IFG impaired fasting glucose, IGT impaired glucose tolerance, BMI body mass index, HDL-C high-density lipoprotein cholesterol, ACE American College of Endocrinology Nathan DM, et al. Diabetes Care. 2007;30:753-759. American College of Endocrinology Task Force on Pre-Diabetes. Available at: www.aace.com/meetings/consensus/hyperglycemia/hyperglycemia.pdf. Accessed November 1, 2008.

Diabetes Prevention Program Don’t Forget: Lifestyle Is More Effective Than Metformin Cumulative Incidence of Diabetes (%) 40 Placebo 30 Metformin 20 Lifestyle 10 0 0 1 2 3 4 Year P 0.001 for each comparison. s *Decrease in risk of developing diabetes compared to placebo group. Diabetes Prevention Program Research Group. N Engl J Med. 2002;346:393-403. Weight Decrease loss in risk* 0.1 kg 2.1 kg 31% 5.6 kg 58%

Case Study Month 2—MD Visit 2 Weight: 294.5 lbs, blood pressure: 140/90, heart rate: 64 bpm, waist: 47 inches Followed diet very strictly for first few weeks – Now on diet 70% of the time – Still skips breakfast Patient rescheduled sleep study, reminded of importance by MD – Reports being very fatigued and realizes he eats to stay awake Action plan – Increase metformin 500-mg to BID, eat protein breakfast instead of skipping the meal

Case Study Sleep-Study Results Apnea-hypopnea index (AHI) of 57.8 – Diagnosis: severe obstructive sleep apneahypopnea syndrome Action plan – Began continuous positive airway pressure (CPAP) treatment with 12 cm H20 Follow-up AHI of 5.0

Clinical Pearl Many patients won’t tolerate CPAP Risk of erectile dysfunction can be a strong motivator

Case Study Month 3—Registered-Dietitian Visit 2 Weight: 290.6 lbs Not eating breakfast – “No time, no interest, not hungry” Eating less at night Patient hurt his back and is going to physical therapy, but little aerobic activity secondary to fatigue Seeing new psychiatrist who will evaluate medical regimen Plan – Meal replacements for breakfast – Continue low-glycemic index diet (increase vegetables, steak only 1x/week)
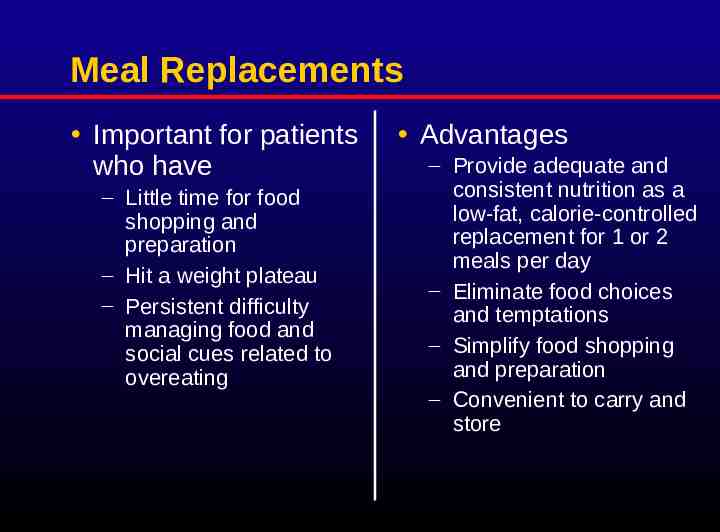
Meal Replacements Important for patients who have – Little time for food shopping and preparation – Hit a weight plateau – Persistent difficulty managing food and social cues related to overeating Advantages – Provide adequate and consistent nutrition as a low-fat, calorie-controlled replacement for 1 or 2 meals per day – Eliminate food choices and temptations – Simplify food shopping and preparation – Convenient to carry and store

Meal Replacements Promote Short- and Long-Term Weight Loss Phase 2 Phase 1* Weight Loss (%) MR-1 CF 0 5 MR-2 10 15 0 2 4 6 8 10 12 18 Time (mo) 24 *1200–1500 kcal/d diet prescription CF conventional foods; MR-2 replacements for 2 meals, 2 snacks daily; MR-1 replacements for 1 meal, 1 snack daily Fletchner-Mors, et al. Obes Res. 2000;8:399. 30 36 45 51

Case Study Month 5—MD Visit 3 Weight: 277.7 lbs (-28 lbs), BMI: 37.7 kg/m2; blood pressure: 130/80, heart rate: 64 bpm, waist: 44 inches Now on CPAP at 12 cm H20 – Notes that he feels much better, with more energy and focus Since sleep study and CPAP use, psychiatrist decreased lithium to 600-mg QD, paroxetine to 20-mg QD, and amphetamine/ dextroamphetamine to 20-mg QD DHA/EPA docosahexaenoic acid/eicosapentaenoic acid

Case Study Month 5—MD Visit 3 Lab Results Glucose: 92 mg/dL TC: 176 mg/dL HDL-C: 30 mg/dL LDL-C: 106 mg/dL TG: 200 mg/dL Non–HDL-C: 146 mg/dL hs-CRP: 3.1 mg/L A1c: 5.6% Creatinine: 1.2 mg/dL AST: 25 U/L ALT: 40 U/L TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglycerides, hsCRP high-sensitivity C-reactive protein, AST aspartate aminotransferase, ALT alanine aminotranferase

ARS Question Which of the following would you be most likely to consider as part of the action plan for this visit? A. Increase statin dosage B. Switch to a different statin C. Add a fibrate D. Discontinue niacin and add omega-3 FAs

Case Study Month 5—MD Visit 3 Action plan Discontinue niacin Start omega-3 (DHA/EPA) fatty acids (FA) 2000-mg QD, to BID Increase metformin to 850-mg BID Clinical Pearl Due to the negative effect of niacin on glucose control and insulin resistance1,2, omega-3 fatty acids may be a preferred alternative in patients at risk for diabetes* *Reflects opinion of program Steering Committee. TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglycerides, hsCRP high-sensitivity C-reactive protein, AST aspartate aminotransferase, ALT alanine aminotranferase 1. Vittone F, et al. J Clin Lipidol. 2007;1:203-210. 2. Goldberg RB, et al. Mayo Clin Proc. 2008; 83:470-8.

Case Study Month 6—Registered-Dietitian Visit 3 Weight: 274.6 lbs Patient has been doing well with breakfast meal replacements, but is bored with diet and feels he has hit a weight plateau Plan – Congratulate him on losing 30 lbs! – Continue low-glycemic index diet, but brainstorm alternative breakfast and snack options – Food records 3 days/week, self-monitor weight every day for next 2 weeks – Reinforce need for physical activity

Case Study Month 8—MD Visit 4 Weight: 270.7 lbs (-35 lbs [-11%]), blood pressure: 124/82, heart rate: 68 bpm, waist: 43 inches Current meds: atorvastatin 10-mg QD, metformin 850mg BID, omega-3 fatty acids (DHA/EPA) 2000-mg BID, ramipril 5-mg BID, amphetamine/dextroamphetamine 20mg QD, paroxetine 20-mg QD, lithium 600-mg QD Using CPAP regularly and has good energy level Fair compliance to diet secondary to stress/family – Has some night eating, but generally minimizing sugar and carbohydrates He now feels active enough to exercise and is walking 20 min/day 4x/week

Case Study Month 8—MD Visit 4, Laboratory Results Glucose: 90 mg/dL TC: 157 mg/dL HDL-C: 42 mg/dL LDL-C: 91 mg/dL TG: 120 mg/dL Non–HDL-c: 115 mg/dL A1c: 5.2% hs-CRP: 1.2 mg/L TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglycerides, hs-CRP high-sensitivity C-reactive protein

Case Study Month 8—MD Visit 4, Action Plan Psychiatrist stopped lithium, reduced paroxetine to 10-mg QD and reduced amphetamine/dextroamphetamine to 10mg QD Continue metformin 850-mg BID and use of CPAP Prescribe exercise regimen

Clinical Pearl In addition to lifestyle factors, biology favors weight regain Eckel RH. N Engl J Med. 2008;358:1941-1950.

ARS Question According to the US Department of Health and Human Services 2008 guidelines, how many minutes per week of moderate-intensity exercise do many people need to maintain their weight after a significant amount of weight loss? A. 60 B. 120 C. 180 D. 300 US Department of Health and Human Services. Available at: http://www.health.gov/paguidelines/guidelines/default.aspx. Accessed February 6, 2009.

Clinical Pearl Although caloric restriction is the key to weight loss, regular physical activity is crucial to maintaining a lower body weight

National Weight Control Registry: Cardinal Behaviors of Successful Long-Term Weight Management Self-monitoring – Diet: record food intake daily, limit certain foods or food quantity – Weight: check body weight 1x/week Low-calorie, low-fat diet – Total energy intake: 1300–1400 kcal/day – Energy intake from fat: 20%–25% Eat breakfast daily Regular physical activity: 2500–3000 kcal/week (eg, walk 4 miles/day) Klem, et al. Am J Clin Nutr. 1997;66:239. McGuire, et al. Int J Obes Relat Metab Disord.1998;22:572.

Key Learnings: Medical Look for sleep apnea and treat it Get your patients off drugs that cause obesity (when possible) Consider insulin sensitizers Assess medications for aggravation of comorbidities Ask patients how well they are sticking to their intended lifestyle changes

Key Learnings: Behavioral Adapt the diet to your patient Inform patients that breakfast is associated with weight loss/lower bodyweight Encourage self-monitoring – Food records – Regular “weigh-ins” Reinforce that exercise is critical for the maintenance of weight loss

At the initial clinical presentation, would this patient have been a candidate for bariatric surgery? Weight: 305.7 lbs, BMI: 41.5 kg/m2, waist: 48 inches, blood pressure: 138/90, heart rate: 68 bpm Patient at his highest weight and gaining – Several weight-loss attempts without significant progress Hyperlipidemia, hypertension, asthma, attention-deficit/hyperactivity disorder, fatigue, depression, obstructive sleep apnea Family history of obesity, type 1 and 2 diabetes Laboratory Test Results TC: 184 mg/dL Non–HDL-C: HDL-C: 33 mg/dL mg/dL LDL-C: 103 mg/dL Glucose: TG: 240 mg/dL mg/dL A1c: hs-CRP: BMI body mass index 151 106 5.9% 8.2 mg/L

Bariatric Surgery Indications – BMI 40 kg/m2 or BMI 35–39.9 kg/m2 and lifethreatening cardiopulmonary disease, severe diabetes, or lifestyle impairment – Failure to achieve adequate weight-loss with nonsurgical treatment Contraindications – History of noncompliance with medical care – Certain psychiatric illnesses: personality disorder, uncontrolled depression, suicidal ideation, substance abuse – Unlikely to survive surgery Adapted from www.obesityonline.org. NIH Consensus Development Panel. Ann Intern Med. 1991;115:956.

Clinical Pearl Surgeon experience is the single best predictor of success To locate an ASMBS Center of Excellence http://www.surgicalreview.org/ ASMBS American Society of Metabolic and Bariatric Surgery.

ARS Question Which of the following is true about the effects of bariatric surgery? A. It has not yet been associated with a significant improvement in overall mortality B. At 10-years postprocedure, it is associated with a decrease in the incidence of hypertension C. At 10-years postprocedure, over 1/3 of patients with diabetes at baseline no longer had the disease

Swedish Obese Subjects Study Bariatric Surgery: Long-Term Effects on Weight and Cardiovascular Risk Factors Prospective, controlled intervention trial of 4047 obese subjects (age 48 years, BMI 41 kg/m2); gastric surgery* vs conventional treatment At 10 years – Weight change—surgery: 16.1% – Weight change—control: 1.6% (P 0.001) – Lower incidence of diabetes, hypertriglyceridemia, and hyperuricemia (P 0.05 for each) Rate of Recovery (% of Subjects) 100 † 73 80 † 60 40 53 48 36 ‡ 24 Hypertriglyceridemia † Surgery † 46 13 20 0 Control Low HDL Cholesterol Sjostrom L, et al. N Engl J Med. 2004;351:2683-2693. Diabetes 11 27 19 Hypertension Hyperuricemia *Banding, vertical-banded gastroplasty, gastric bypass † P 0.001 ‡ P 0.02

Swedish Obese Subjects Study Bariatric Surgery: Long-Term Weight Loss and Decreased Mortality Up to 16 years follow-up Overall mortality – Hazard ratio* 0.76 (95% CI: 0.59–0.99), P 0.04 Cumulative Mortality Mortality (%) (%) Cumulative Change in Weight (%) 00 Control Control 10 10 Banding Banding -20 -20 Vertical-Banded Vertical-Banded Gastroplasty Gastroplasty Gastric Gastric Bypass Bypass -30 -30 14 14 12 12 Control Control 10 10 88 66 Surgery Surgery 44 P P 0.04 0.04 22 00 00 11 22 33 44 66 88 10 10 Years Years *Surgical group vs control group at 16 years Sjostrom L, et al. N Engl J Med. 2007;357:741-752. 15 15 00 22 44 66 88 Years Years 10 10 12 12 14 14 16 16

Key Learnings: Bariatric Surgery Advantages – “Forced” lifestyle changes – Improved cardiometabolic risk-factors – Decrease in diabetes Both recovery and incidence – Decrease in mortality Pitfalls – Surgical complications – “Forced” lifestyle changes – Patients can “get around” the surgery






