Heating / Cooling Curve Calculations EQ: Why is an ideal heating
17 Slides125.53 KB

Heating / Cooling Curve Calculations EQ: Why is an ideal heating curve not a straight line graph?
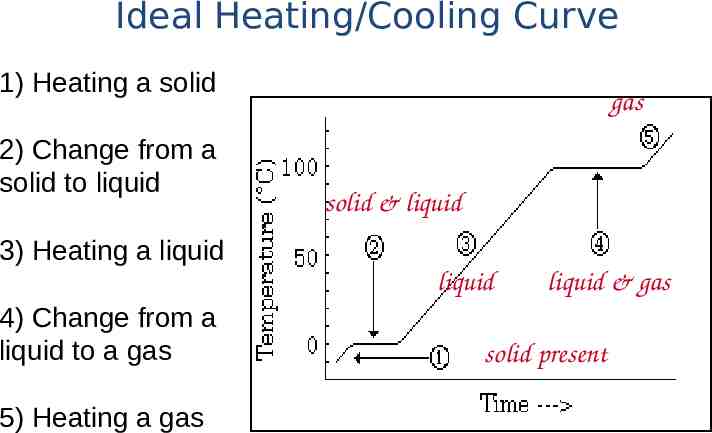
Ideal Heating/Cooling Curve 1) Heating a solid 2) Change from a solid to liquid gas solid & liquid 3) Heating a liquid liquid 4) Change from a liquid to a gas 5) Heating a gas liquid & gas solid present
On the back of you notes write:
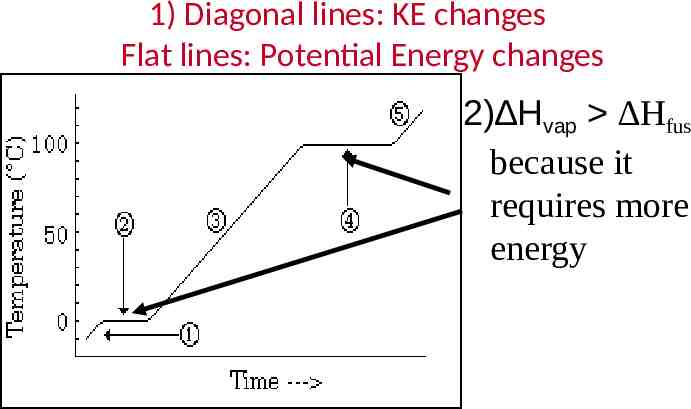
1) Diagonal lines: KE changes Flat lines: Potential Energy changes 2) Hvap Hfus because it requires more energy
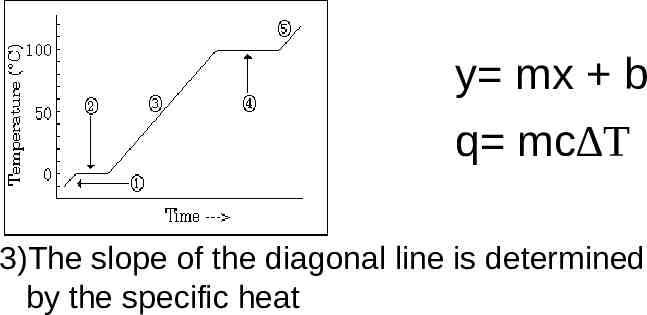
y mx b q mc T 3)The slope of the diagonal line is determined by the specific heat
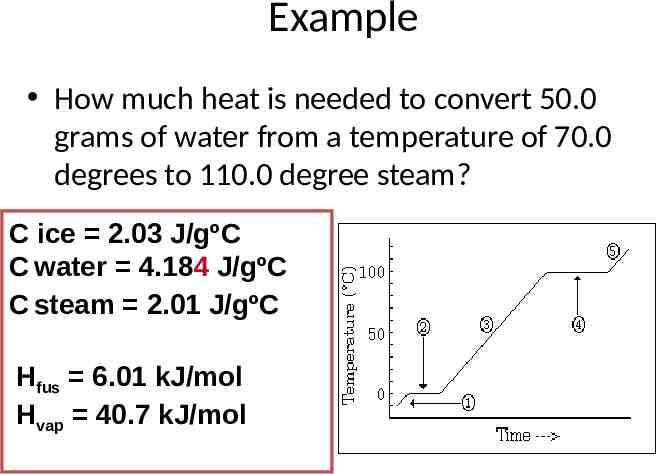
Example How much heat is needed to convert 50.0 grams of water from a temperature of 70.0 degrees to 110.0 degree steam? C ice 2.03 J/gºC C water 4.184 J/gºC C steam 2.01 J/gºC Hfus 6.01 kJ/mol Hvap 40.7 kJ/mol
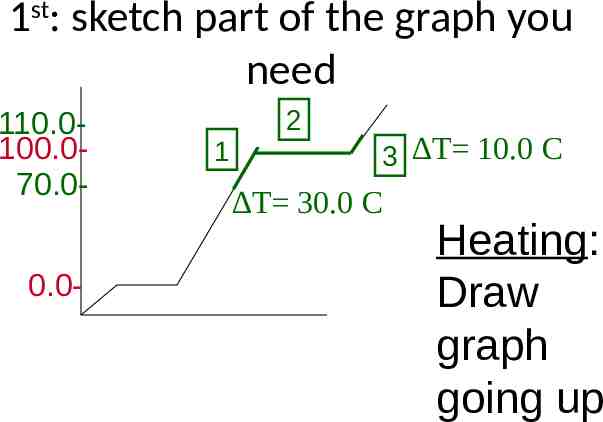
1st: sketch part of the graph you need 110.0100.070.00.0- 1 2 C 3 T 10.0 T 30.0 C Heating: Draw graph going up
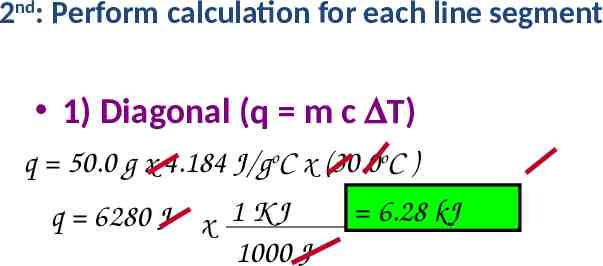
2nd: Perform calculation for each line segment 1) Diagonal (q m c DT) q 50.0 g x 4.184 J/goC x (30.0oC ) q 6280 J x 1 K J 1000 J 6.28 kJ
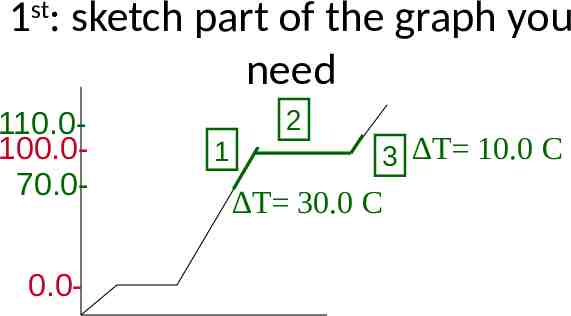
1st: sketch part of the graph you need 110.0100.070.00.0- 1 2 C 3 T 10.0 T 30.0 C
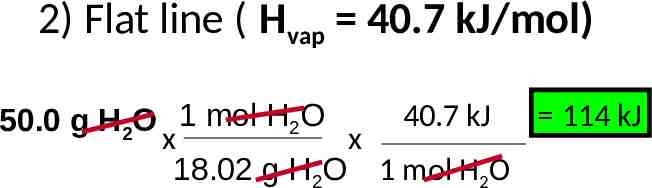
2) Flat line ( Hvap 40.7 kJ/mol) 40.7 kJ 114 kJ 50.0 g H2O 1 mol H2O x x 18.02 g H2O 1 mol H2O
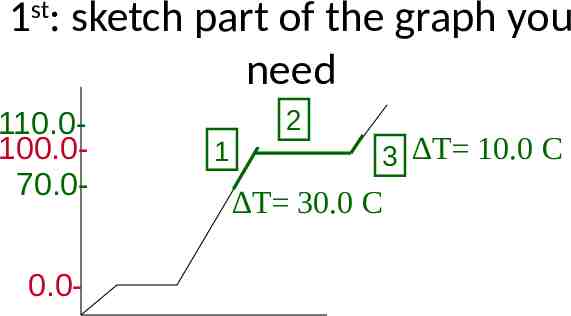
1st: sketch part of the graph you need 110.0100.070.00.0- 1 2 C 3 T 10.0 T 30.0 C
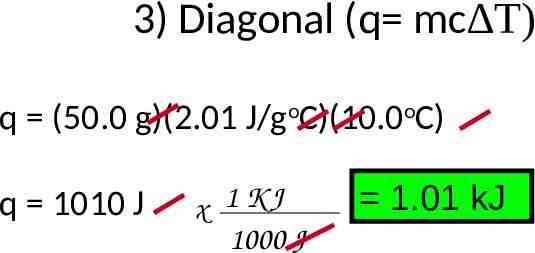
3) Diagonal (q mc T) q (50.0 g)(2.01 J/goC)(10.0oC) q 1010 J x 1 KJ 1000 J 1.01 kJ
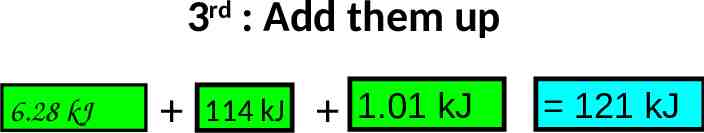
3 : Add them up rd 6.28 kJ 114 kJ 1.01 kJ 121 kJ
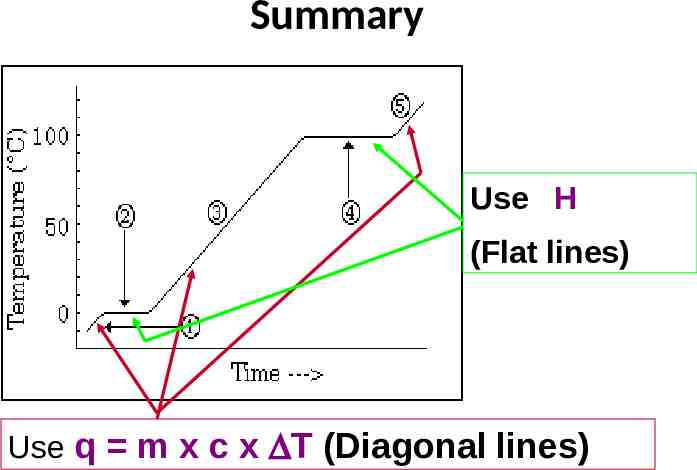
Summary Use H (Flat lines) Use q m x c x DT (Diagonal lines)
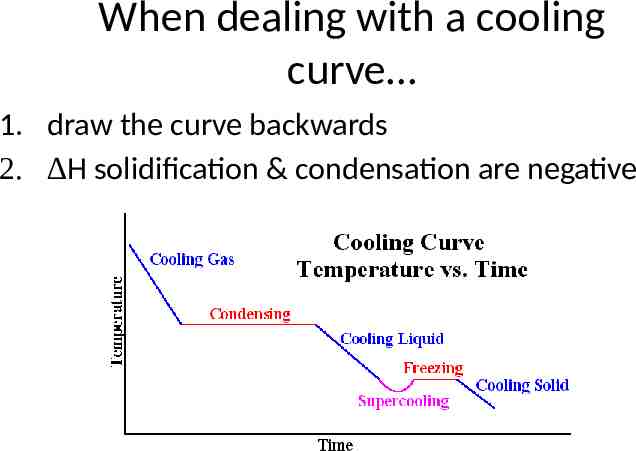
When dealing with a cooling curve 1. draw the curve backwards 2. H solidification & condensation are negative

Notebook Problems 1. Calculate the amount of heat needed to convert 10.0 grams of ice from a temperature of -23.0oC to water at 27.0oC. 2. Calculate the amount of heat released when 50.0 grams of steam at a temperature of 123.0oC cools into water at 77.0oC.
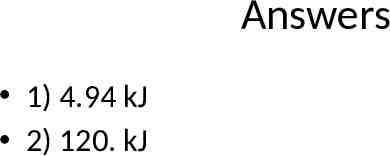
Answers 1) 4.94 kJ 2) 120. kJ






