Tips for Implementing Your Health Center Quality Improvement
64 Slides1.97 MB

Tips for Implementing Your Health Center Quality Improvement Program Bureau of Primary Health Care Health Resources and Services Administration Department of Health and Human Services March 31, 2011

Introduction Quality is a focus area nationally and at HRSA Assessment of QI plans showed areas for improvement Invest in your QI infrastructure – Clinical quality and beyond Focus on implementation – This work never ends
Benefits of an Effective QI Plan Roadmap for HC organization – Leadership, focus, & prioritization – Efficient coordination of staff & resources – Better outcomes Satisfy external requirements – HRSA, State – Third-party quality accreditation and recognition 3

How Can HRSA Help? Support for planning and implementation of Quality Improvement strategies – – – – QI Plan Learning Series Further guidance Resources and technical assistance Third-party quality recognition How else can we help you with your QI plan and other quality efforts?

Office of Quality & Data Activities Federal Tort Claims Act deeming – Health centers and free clinics – ECRI resources Data collection and analysis – UDS, patient survey, EHB Health Information Technology – Adoption, meaningful use, health info exchange Quality – Third party quality recognition – Aligning technical assistance for PCMH transformation – CMS

Accreditation Effort to gain 3rd party recognition by TJC/AAAHC to ensure quality in the HCs In January 2011, 291 centers (25.7%) were accredited

PCMH The Bureau is committed to harnessing the known benefits of the patient centered medical home Currently, two major PCMH activities are being conducted: – Patient Centered Medical Health Home Initiative or PCMHHI (HRSA) – Advanced Primary Care Practice Medicare demo in FQHCs (CMS)

PCMHHI 351 NOIs, 341 are 1st time (through 2/17/11) NCQA has 1st 100, preparing to enter into survey recognition process Single application for grantee for single or multiple sites within organization NCQA to provide T.A. to guide through survey process 5 percent on-site follow up

Medicare Demo: Advanced Primary Care Practice Run from CMS with HRSA support Rolling application for up to 500 HCs, having seen 200 unique Medicare beneficiaries in past 12 months Will be required to achieve NCQA level III in 3 years

Technical Assistance To promote transformation, HRSA is working with CMS on a T.A. strategy Readiness assessment and gap analysis critical first step T.A. could include practice coaching, peer to peer learning, data/evaluation and knowledge management PCAs and states will have high stake, be well situated to craft T.A. delivery for grantees

Using HIT and Data in Your QI Program Different types of measures: clinical, patient experience, staff satisfaction, and financial domains UDS, patient experience surveys, dental and oral health measures, and other data sources Tips on useful EHB reports Involving Health Information Exchange goals in your QI plan 11

TIPS FOR IMPLEMENTING YOUR QUALITY PLAN HRSA Quality Series #3 Jan Wilkerson, RN, CPHQ, GAPHC 12

“Quality is never an accident; It is always the result of high intention, sincere effort, intelligent direction, and SKILLFUL EXECUTION. It represents the wise choice of many alternatives.” William A. Foster 13

Objectives Participants will be able to: Evaluate the FQHC’s current Quality Plan Identify quality policies needed to develop and support the quality infrastructure Determine the best skillful execution for your FQHC’s Quality Plan Develop a formal process for quality communication including reporting and staff training 14
QUALITY PLAN Assessment 15

Assessment Criteria HRSA FQHC program requirements http://bphc.hrsa.gov/about/requirements.htm HRSA Scope of Project; defines the capacity of services provided by the FQHC http://bphc.hrsa.gov/policiesregulations/policies/index.html HRSA KEY HEALTH CENTER PROGRAM REQUIREMENTS http://bphc.hrsa.gov/about/requirements.htm FTCA requirements (42 CFR 51c.303(c)(1-2) http://bphc.hrsa.gov/policiesregulations/policies/index.html (Section 330(k)(3)(C) of the PHS Act, 45 CFR Part 74.25 (c)(2), (3) and 42 CFR Part 51c.303(c)(1-2)) The Health Care Quality Improvement Act of 1986, as amended 42 USC Sec. 11101 01/26/98 STATE Georgia Code #3910 (GA. L. 1975) Accreditation requirements 16

Other Considerations Measures should include: Required measures Deficient goals/ benchmarks New/ revised processes Business Plan & Health Care Plan measures High volume High risk Problem-prone Satisfaction survey The FQHC’s mission and Strategic Plan Sub-committees and workgroups Quality methodology Quality Policies HRSA GRANT Your Business Plan & Health Care Plan 17
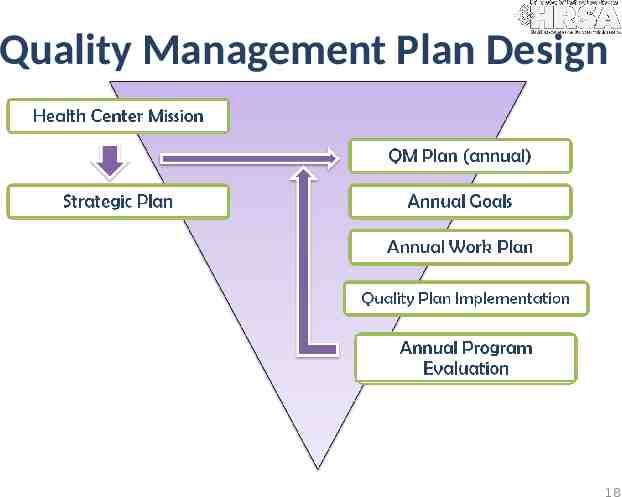
Quality Management Plan Design 18

“ we're on our way We've only just begun ” The Carpenters 20
PLANNING FOR SUCCESS Infrastructure 21
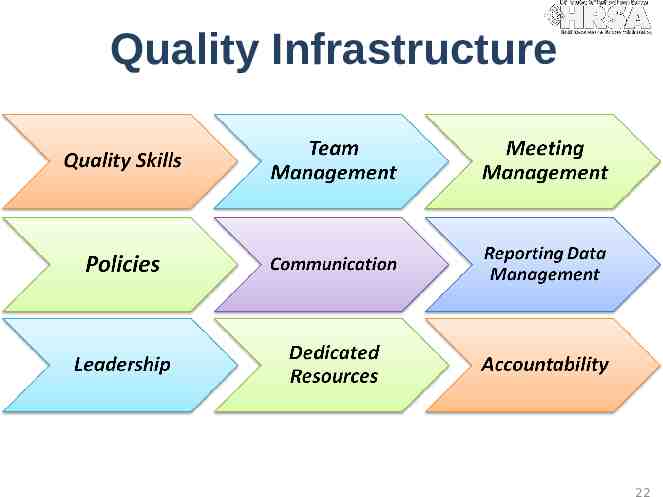
Quality Infrastructure 22
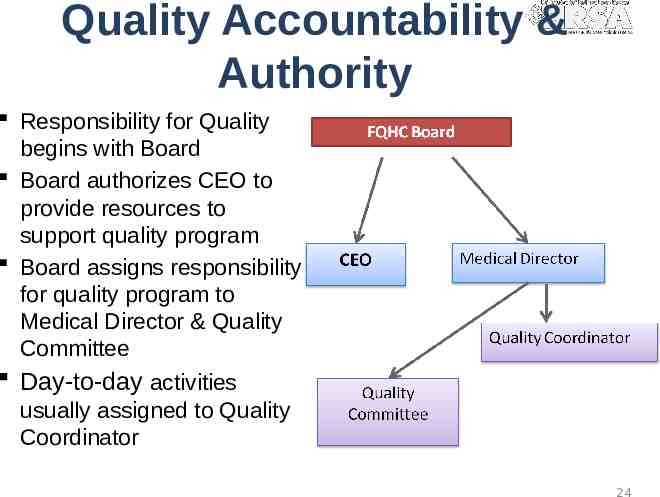
Quality Accountability & Authority Responsibility for Quality begins with Board Board authorizes CEO to provide resources to support quality program Board assigns responsibility for quality program to Medical Director & Quality Committee Day-to-day activities usually assigned to Quality Coordinator 24
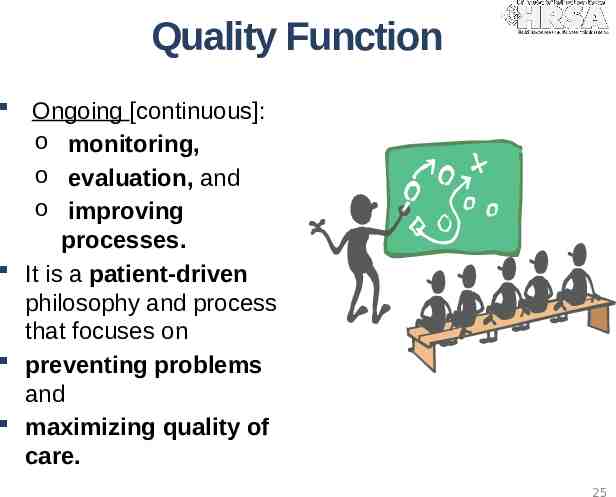
Quality Function Ongoing [continuous]: o monitoring, o evaluation, and o improving processes. It is a patient-driven philosophy and process that focuses on preventing problems and maximizing quality of care. 25

Quality Committee Members Chair – Medical Director or Physician designee QI Coordinator (facilitator) Risk/ ComplianceSafety Coordinator Medical Staff Clinical Staff Finance Front Office Medical Records In-house Lab Staff All departments / services represented All sites represented 26
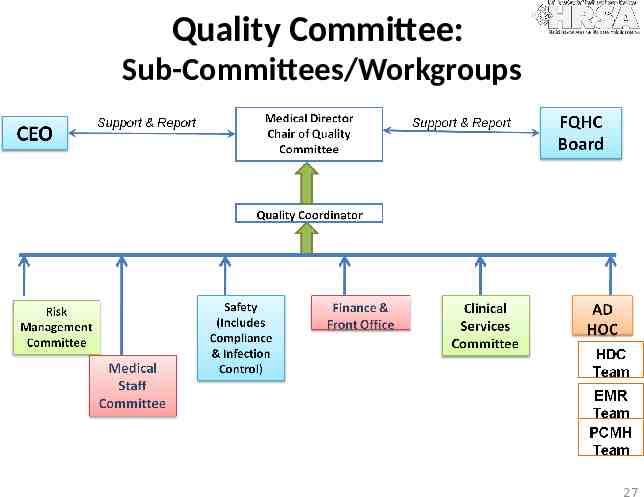
Quality Committee: Sub-Committees/Workgroups 27

Quality Committee: Sub-Committees/ Workgroups Medical Staff Finance (Business Committee – privileging, peer review Safety Compliance Infection Control Risk Management Utilization Management Plan) Work groups – HDC Teams or other Quality Initiatives EMR Work group Clinical Work group Ad Hoc work groups Indicates necessary regardless of FQHC size 29

QUALITY COMMITTEE Role & Responsibility 30

Expectations of the Quality Committee Prioritizing current quality initiatives and activities Quality assessment and planning and annual program evaluation Subcommittee and team chartering, with accountability & reporting followed up by the QC The ongoing monitoring, evaluation, and improvement of processes and system. With a patient-driven philosophy and process that focuses on preventing problems and maximizing quality of care. 31

Key Aspects & Functions of Quality Management Policies & Procedures Medical Record Clinical Protocols Tracking Systems Trend identification that impact systems and processes Quality Planning Credentialing Peer Review Privileging Compliance Safety Risk Management 32
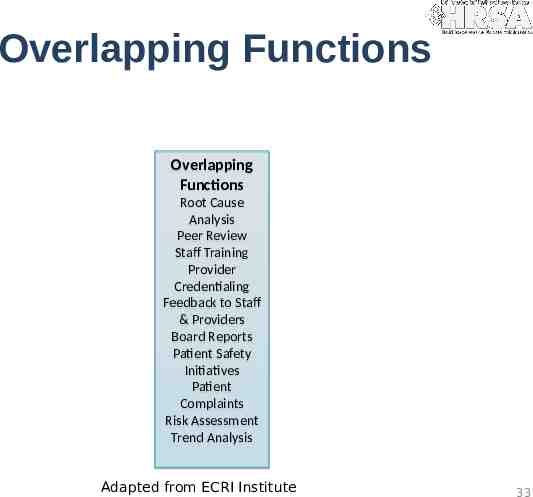
Overlapping Functions Overlapping Functions Root Cause Analysis Peer Review Staff Training Provider Credentialing Feedback to Staff & Providers Board Reports Patient Safety Initiatives Patient Complaints Risk Assessment Trend Analysis Adapted from ECRI Institute 33

Skills for Quality Teams Confidential nature of quality improvement Understanding of the Quality Plan and policies to support the quality program Know their role and responsibilities as a team member and as a leader/ cheerleader of quality Engaged as process “owners” for the organization not just my corner of it PDSA methodology & Care Model understanding Understanding data and measurement Knowledge of process improvement Basic toolkit – graphs, auditing, required measures 35
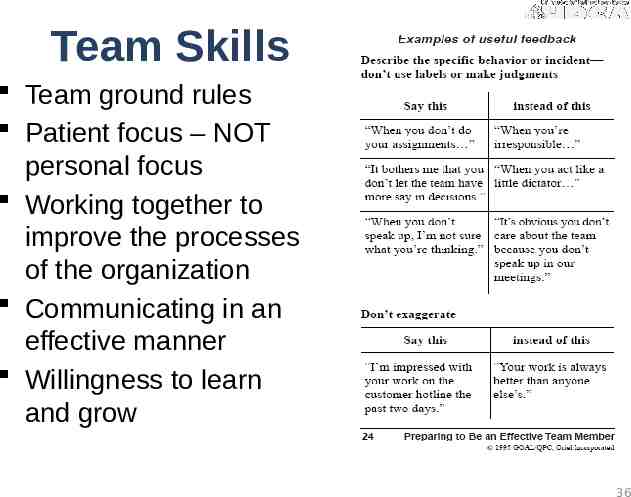
Team Skills Team ground rules Patient focus – NOT personal focus Working together to improve the processes of the organization Communicating in an effective manner Willingness to learn and grow 36

Meeting Skills Agendas Minutes (no PHI – ever) Staying on point Start and end on time – regardless of who is/isn’t there 100-mile rule (no interruptions/ cell phones) Accountability from the group for assigned tasks Templates and examples available upon request and in GAPHC Quality Manuals- http://www.gaphc.org 38
Quality Methodology Data Management & Analysis 39

Brainstorming 40
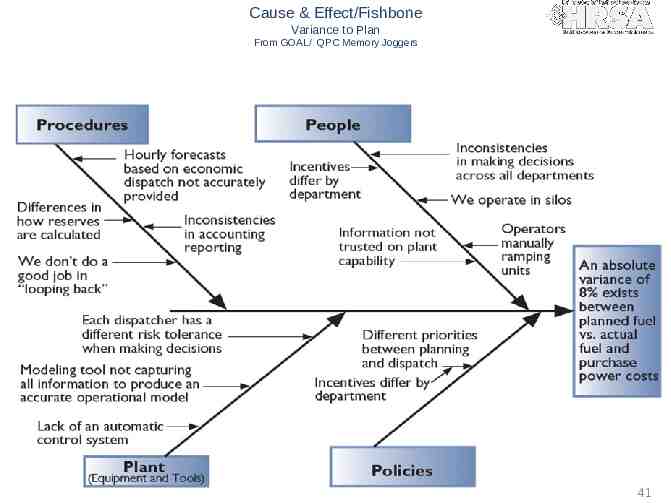
Cause & Effect/Fishbone Variance to Plan From GOAL/ QPC Memory Joggers 41

Improving Processes From The Team Handbook 43

Benchmarking & Goals National Benchmark State Benchmark http://www.hrsa.gov/data-statistics/health-centerdata/StateData/index.html FQHC previous year(s) FQHC previous audits current year Performance Goal Pre-set Trigger? 44

Using and Managing Data Reporting

Confidentiality Protected & Privileged The Health Care Quality Improvement Act of 1986, as amended 42 USC Sec. 11101 01/26/98. Note each state has legislation defining confidentiality and protection for individuals carrying out quality improvement activities Specific requirements for maintaining confidentiality in the organization Signed Attestation Policy Excellent way to remind everyone of the importance of protecting Confidentiality Some accreditation agencies require it At minimum, Board, QI staff and Quality Committee members, place in HR file 46

QUALITY INFRASTRUCTURE Policies & Processes 47
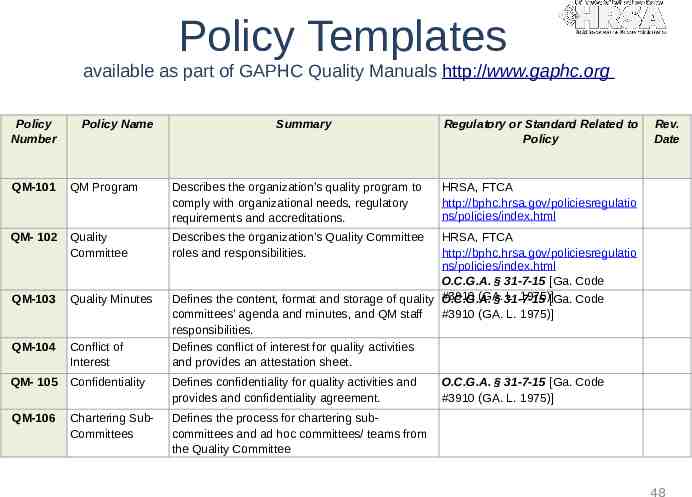
Policy Templates available as part of GAPHC Quality Manuals http://www.gaphc.org Policy Number Policy Name Summary QM-101 QM Program Describes the organization’s quality program to comply with organizational needs, regulatory requirements and accreditations. QM- 102 Quality Committee Describes the organization’s Quality Committee roles and responsibilities. QM-103 Quality Minutes QM-104 Conflict of Interest QM- 105 Confidentiality Defines confidentiality for quality activities and provides and confidentiality agreement. QM-106 Chartering SubCommittees Defines the process for chartering subcommittees and ad hoc committees/ teams from the Quality Committee Regulatory or Standard Related to Policy Rev. Date HRSA, FTCA http://bphc.hrsa.gov/policiesregulatio ns/policies/index.html HRSA, FTCA http://bphc.hrsa.gov/policiesregulatio ns/policies/index.html O.C.G.A. § 31-7-15 [Ga. Code (GA. L. 1975)][Ga. Code Defines the content, format and storage of quality #3910 O.C.G.A. § 31-7-15 committees’ agenda and minutes, and QM staff #3910 (GA. L. 1975)] responsibilities. Defines conflict of interest for quality activities and provides an attestation sheet. O.C.G.A. § 31-7-15 [Ga. Code #3910 (GA. L. 1975)] 48
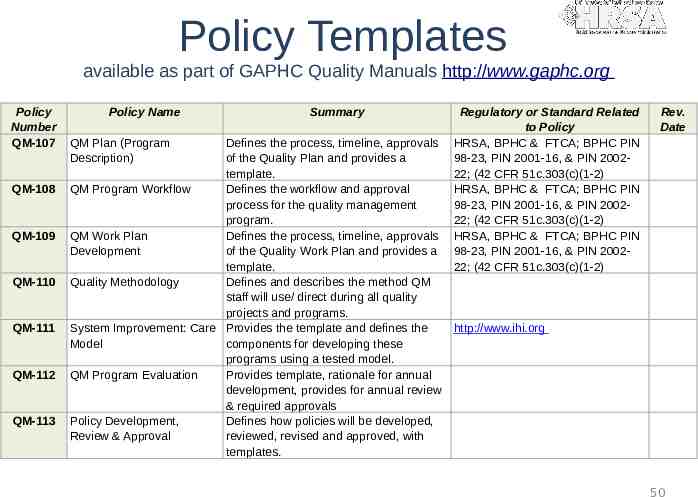
Policy Templates available as part of GAPHC Quality Manuals http://www.gaphc.org Policy Number QM-107 QM-108 QM-109 QM-110 QM-111 QM-112 QM-113 Policy Name QM Plan (Program Description) Summary Defines the process, timeline, approvals of the Quality Plan and provides a template. QM Program Workflow Defines the workflow and approval process for the quality management program. QM Work Plan Defines the process, timeline, approvals Development of the Quality Work Plan and provides a template. Quality Methodology Defines and describes the method QM staff will use/ direct during all quality projects and programs. System Improvement: Care Provides the template and defines the Model components for developing these programs using a tested model. QM Program Evaluation Provides template, rationale for annual development, provides for annual review & required approvals Policy Development, Defines how policies will be developed, Review & Approval reviewed, revised and approved, with templates. Regulatory or Standard Related to Policy HRSA, BPHC & FTCA; BPHC PIN 98-23, PIN 2001-16, & PIN 200222; (42 CFR 51c.303(c)(1-2) HRSA, BPHC & FTCA; BPHC PIN 98-23, PIN 2001-16, & PIN 200222; (42 CFR 51c.303(c)(1-2) HRSA, BPHC & FTCA; BPHC PIN 98-23, PIN 2001-16, & PIN 200222; (42 CFR 51c.303(c)(1-2) Rev. Date http://www.ihi.org 50

IMPLEMENTATION Skillful Execution 52
Closing The Measure Loop 53

Performance Measurement This section defines the technique (process of HOW) the organization improves quality. In order to be effective, the process must be systematic with continuous activities that lead to measureable improvement. Included here is: Indicates who will collect and analyze data Indicates who is accountable for collecting, analyzing and reviewing performance data results and communicating findings Includes strategies on how to report and disseminate results and findings; communicate information about quality improvement activities Describe the process to use data to develop new QI activities and address identified gaps This section will stimulate several Quality Policies to be developed to support this document. 55

Performance Measurement Performance measurement is necessary to track progress towards the end goal of improvement. Steps to measure performance: First, identify the critical aspects of the care and services provided Second determine care and services that are: High Risk High Volume Problem Prone Identify indicators to measure these important aspects of care and service to determine organizational performance and identify areas for improvement 56
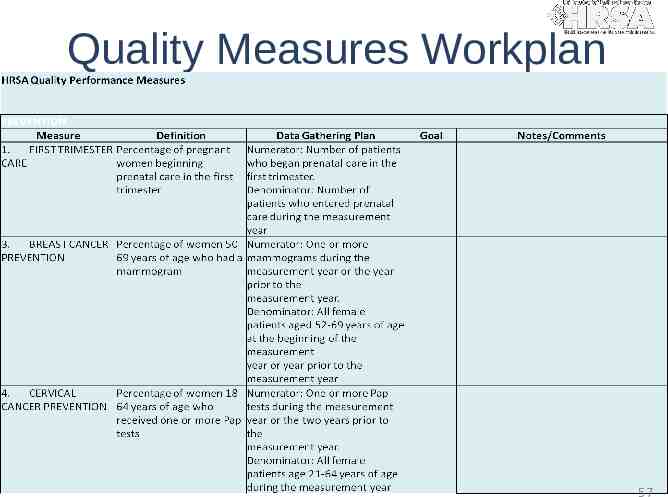
Quality Measures Workplan 57
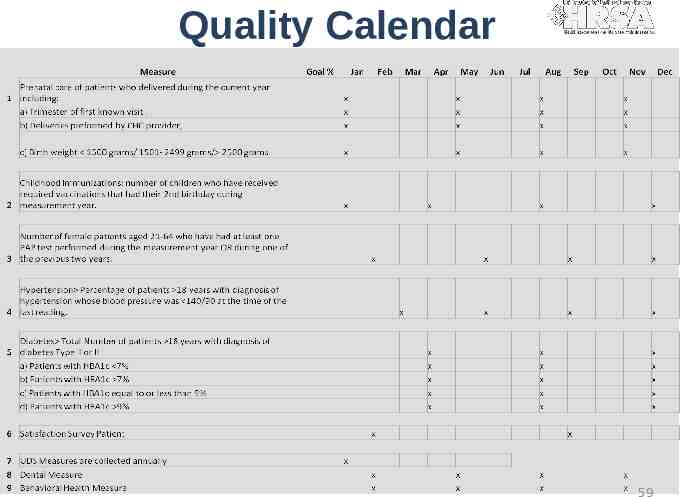
Quality Calendar 59

QUALITY Communication 61
Quality Communication 62
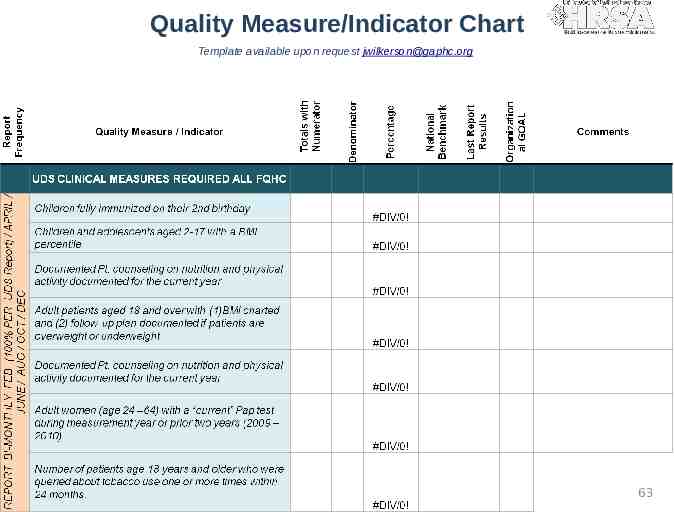
Quality Measure/Indicator Chart Template available upon request [email protected] 63
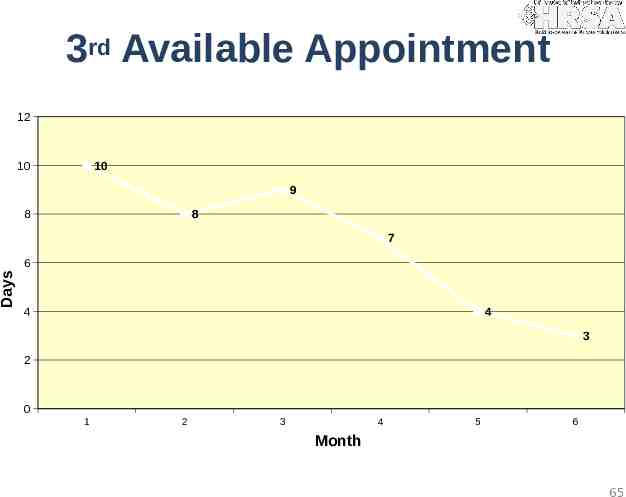
3rd Available Appointment 12 10 10 9 8 8 7 Days 6 4 4 3 2 0 1 2 3 4 5 6 Month 65
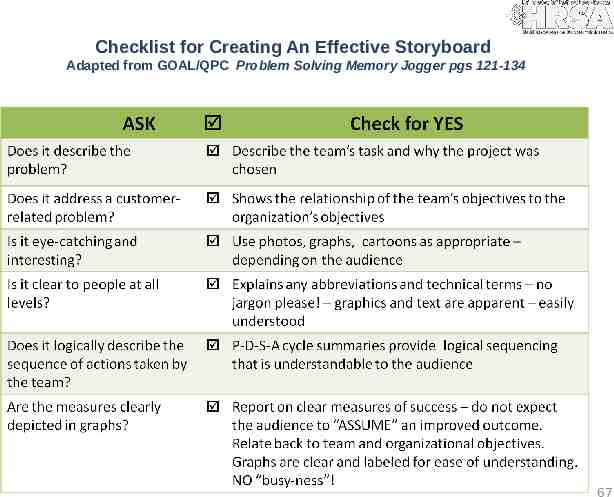
Checklist for Creating An Effective Storyboard Adapted from GOAL/QPC Problem Solving Memory Jogger pgs 121-134 67

“Data is a lot like garbage. You have to know what you are going to do with the stuff BEFORE you start collecting it.” Mark Twain 69

Next Steps 70

Annual Program Evaluation Reference policy and format Summary description of mechanism Reporting process Who is responsible for developing, reviewing and approving Frequency – usually annually 71

Back to PDSA: WORK THE PLAN Step 1: Develop the Quality Improvement Plan; development/ revisions are based on: The organization (system) Population & services provided External requirements (HRSA, accreditation) Step 2: Implement the Quality Improvement Plan Use the QI Plan as the roadmap for implementing an integrated quality program system-wide Step 3: Evaluate the Quality Improvement Plan Did you do what you said you were going to do? Why? Why not? What were the results? How can next year be better? Step 4: Act on the lessons learned to revise the Quality Improvement Plan for the next year 72

Quality Resources The Team Handbook – 3rd Edition; Scholtes/ Joiner/ Streibel : Amazon.com http://www.amazon.com/Team-Handbook-Third-Peter-Scholt es/dp/1884731260 GOAL/ QPC Memory Joggers http://www.goalqpc.com/shop products.cfm?ProductShopB y 7 NAHQ Guide to Quality Management, 8th edition, Handbook for Improvement, 1997 ; http://www.nahq.org/ HRSA Quality Matrix – Performance Measures http://www.hrsa.gov/healthit/coremeasures.html GAPHC Quality Management Plan Template & Manuals , Jan Wilkerson, RN, CPHQ; http://www.gaphc.org 73

Quality Resources NCQA- http://www.ncqa.org/tabid/1196/Default.aspx IHI http://www.ihi.org/IHI/Results/WhitePapers/ AHRQ http://www.ahrq.gov/clinic/epcix.htm Institute for Clinical Systems Improvement http://www.icsi.org/ UDS Manual, -HRSA; http://www.hrsa.gov/data-statistics/health-center-data/rep orting/2010manual.pdf NACHC – http://www.nachc.org ; Information Bulletin #15: Using Information Technology to Improve Quality UDS Benchmark National & State: http://bphc.hrsa.gov/healthcenterdatastatistics/index.html 74

Measures Resources Use nationally [standardized] developed measures, rather than developing your own HRSA Clinical Quality Performance Measures http://www.hrsa.gov/healthit/coremeasures.html Healthy People (2010 & 2020) http://www.healthypeople.gov/About/ National Forum http://www.qualityforum.org/ Agency for Healthcare Quality & Research http://www.ahrq.gov/ NCQA HEDIS Measures http://www.ncqa.org/tabid/59/Default.aspx 75

Resources FTCA for Health Centers: http://bphc.hrsa.gov/FTCA/ FQHC PIN & PALS: http://bphc.hrsa.gov/policiesregul ations/policies/index.html 76

Investigating An Out Of Control Process: Questions to Ask 1. Was a new collection tool/ method used? 2. Were all staff consistent in collection method? 3. Is process affected by new or significant change in environment? 4. Is process affected by predictable conditions? 5. Any new or untrained staff involved in the process during measurement period? 6. Change in resource for input to the process? 7. Is process affected by employee fatigue? 8. Has there been a change in policy/procedure? 9. Is process adjusted frequently? 10. Did sample come from different parts of the process? 11. Are new employees afraid to report? 77

Discussion and Questions Please share your quality improvement successes, challenges, and training and technical assistance needs Contact your HRSA Project Officer or the Office of Quality and Data at [email protected] or (301) 594-0818 78






