Of drug cocktails and inhibited enzymatic rates Why drug cocktails
21 Slides3.15 MB

Of drug cocktails and inhibited enzymatic rates Why drug cocktails are more effective than single-drug therapies, and how to design them Harvard Life Sciences Outreach November 9, 2017

Learning goal and objectives Learning Goal: To understand how structural and biochemical data can be used to rationally design drugs. Learning Objectives: Be able to: Explain how we quantitatively describe “structural similarity” Distinguish “competitive” vs “noncompetitive” enzyme inhibition (structurally and biochemically) Use enzymatic kinetic data to determine which inhibitors would combine to make the best drug cocktail

Learning goal and objectives Learning Goal: To understand how structural and biochemical data can be used to rationally design drugs. Learning Objectives: Be able to: Explain how we quantitatively describe “structural similarity” Distinguish “competitive” vs “noncompetitive” enzyme inhibition (structurally and biochemically) Use enzymatic kinetic data to determine which inhibitors would combine to make the best drug cocktail
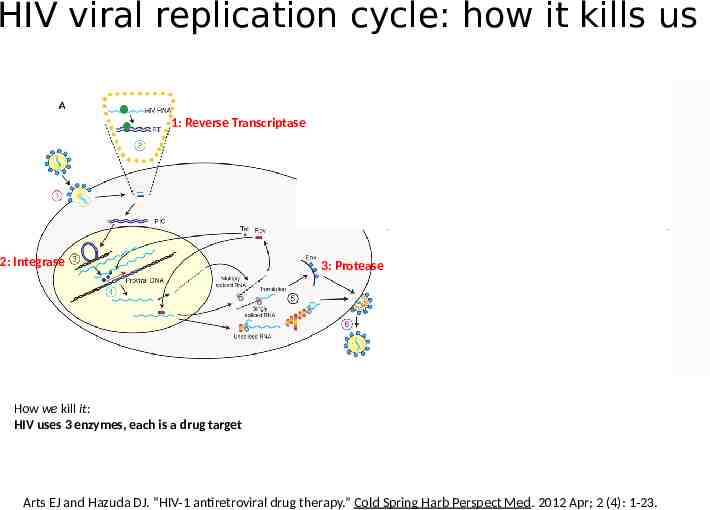
HIV viral replication cycle: how it kills us 1: Reverse Transcriptase 2: Integrase 3: Protease How we kill it: HIV uses 3 enzymes, each is a drug target Arts EJ and Hazuda DJ. “HIV-1 antiretroviral drug therapy.” Cold Spring Harb Perspect Med. 2012 Apr; 2 (4): 1-23.
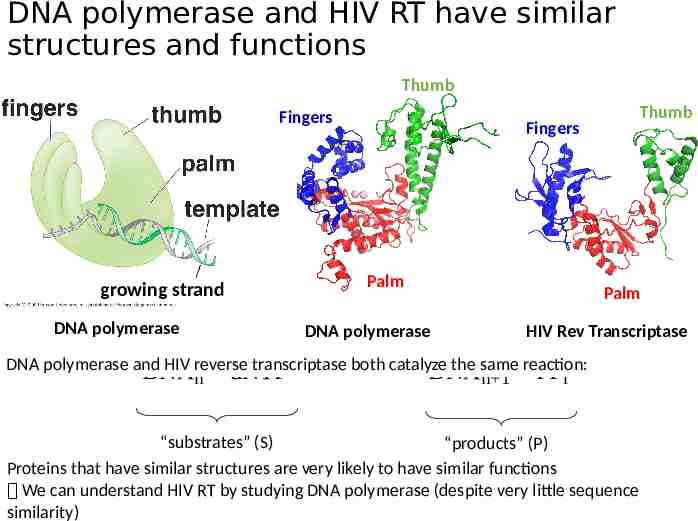
DNA polymerase and HIV RT have similar structures and functions Thumb Fingers growing strand DNA polymerase Thumb Fingers Palm DNA polymerase Palm HIV Rev Transcriptase DNA polymerase and HIV reverse transcriptase both catalyze the same reaction: DNA DNA n dNTP n 1 PPi “substrates” (S) “products” (P) Proteins that have similar structures are very likely to have similar functions We can understand HIV RT by studying DNA polymerase (despite very little sequence similarity)
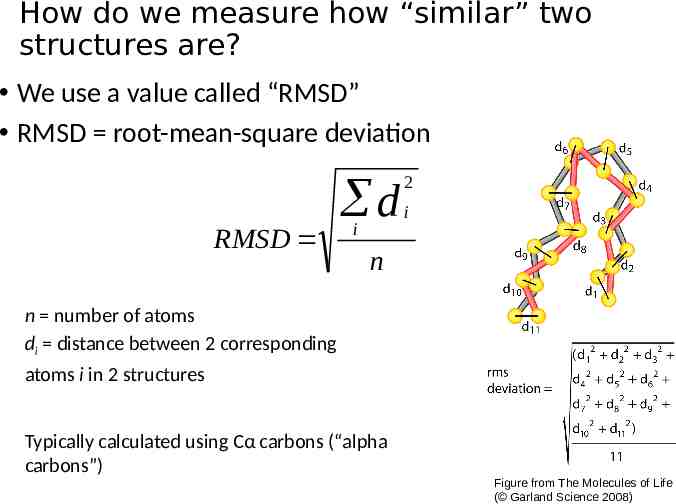
How do we measure how “similar” two structures are? We use a value called “RMSD” RMSD root-mean-square deviation RMSD d i 2 i n n number of atoms di distance between 2 corresponding atoms i in 2 structures Typically calculated using Cα carbons (“alpha carbons”) Figure from The Molecules of Life ( Garland Science 2008)
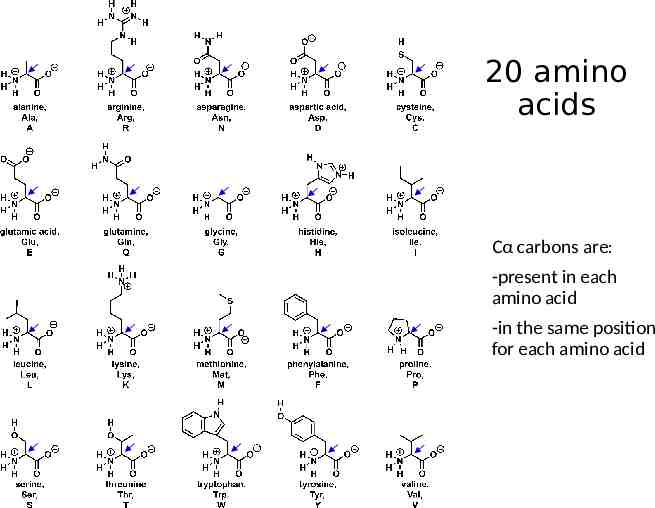
20 amino acids Cα carbons are: -present in each amino acid -in the same position for each amino acid
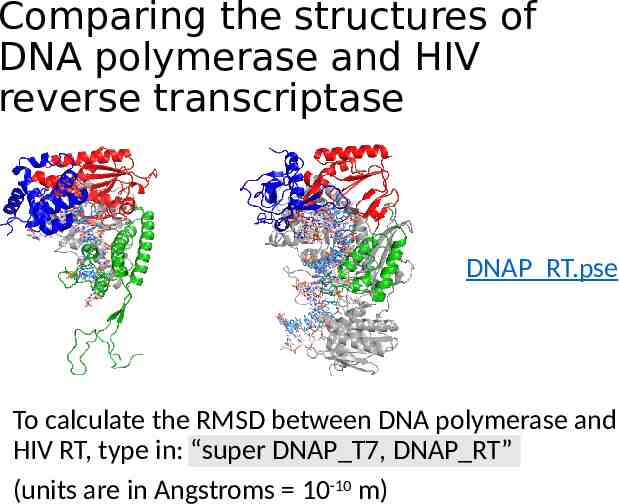
Comparing the structures of DNA polymerase and HIV reverse transcriptase DNAP RT.pse To calculate the RMSD between DNA polymerase and HIV RT, type in: “super DNAP T7, DNAP RT” (units are in Angstroms 10-10 m)
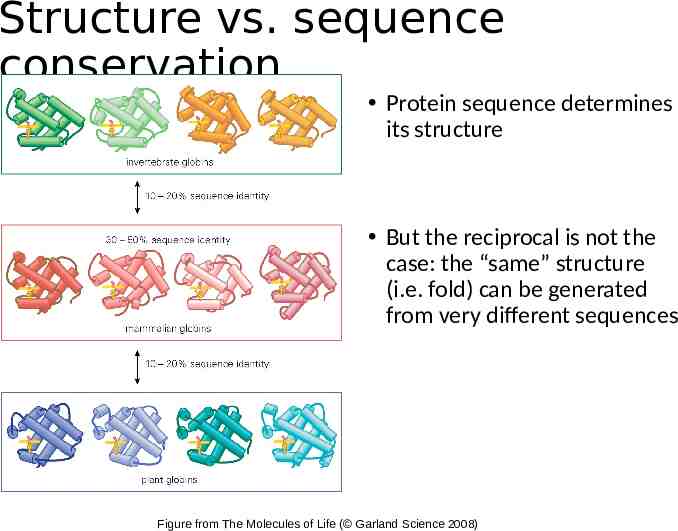
Structure vs. sequence conservation Protein sequence determines its structure But the reciprocal is not the case: the “same” structure (i.e. fold) can be generated from very different sequences Figure from The Molecules of Life ( Garland Science 2008)

Learning goal and objectives Learning Goal: To understand how structural and biochemical data can be used to rationally design drugs. Learning Objectives: Be able to: Explain how we quantitatively describe “structural similarity” Distinguish “competitive” vs “noncompetitive” enzyme inhibition (structurally and biochemically) Use enzymatic kinetic data to determine which inhibitors would combine to make the best drug cocktail
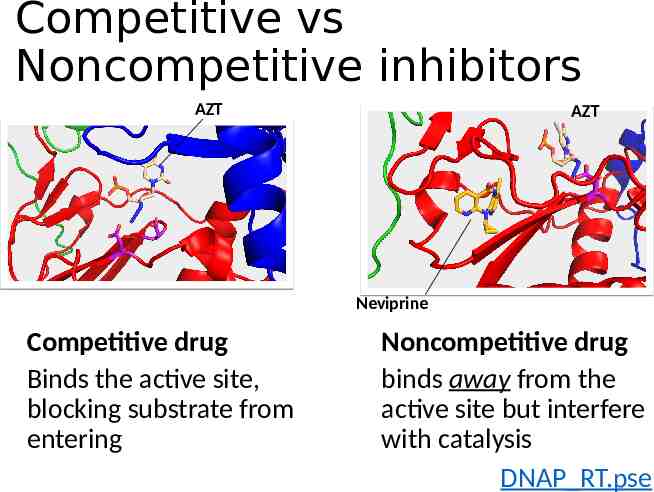
Competitive vs Noncompetitive inhibitors AZT AZT Neviprine Competitive drug Binds the active site, blocking substrate from entering Noncompetitive drug binds away from the active site but interfere with catalysis DNAP RT.pse
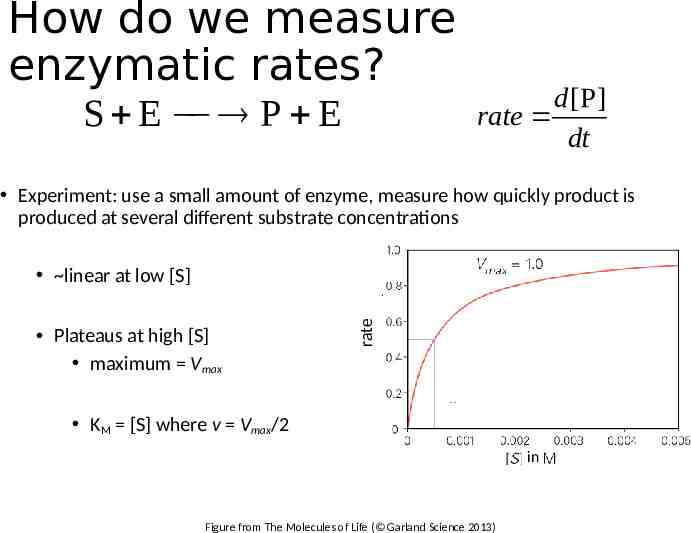
How do we measure enzymatic rates? S E P E d [P] rate dt Experiment: use a small amount of enzyme, measure how quickly product is produced at several different substrate concentrations Plateaus at high [S] maximum Vmax rate linear at low [S] KM [S] where v Vmax/2 in Figure from The Molecules of Life ( Garland Science 2013)
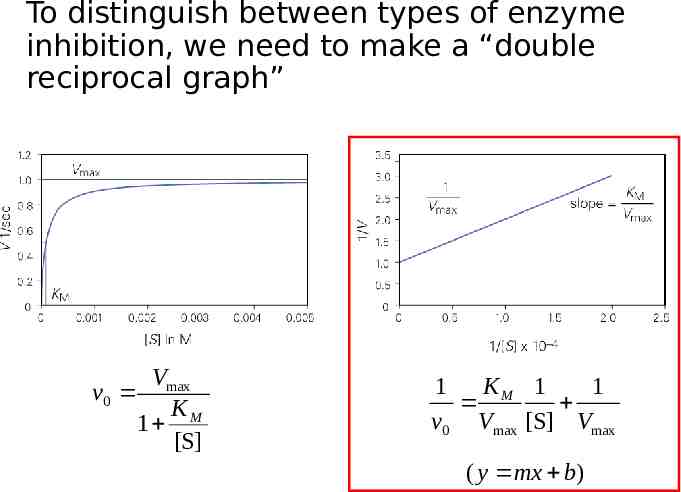
To distinguish between types of enzyme inhibition, we need to make a “double reciprocal graph” V v0 max KM 1 [S] 1 KM 1 1 v0 Vmax [S] Vmax ( y mx b)
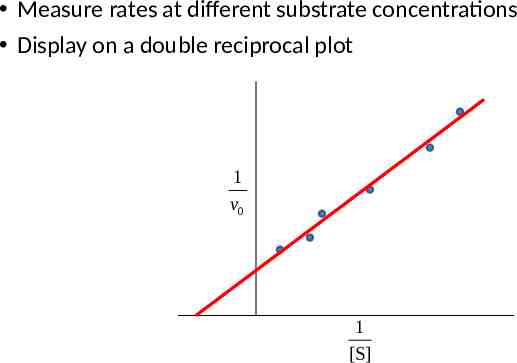
Measure rates at different substrate concentrations Display on a double reciprocal plot 1 v0 1 [S]
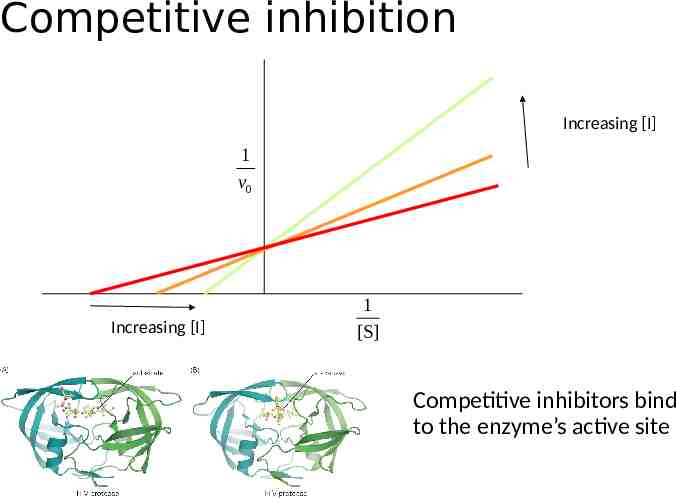
Competitive inhibition Increasing [I] 1 v0 Increasing [I] 1 [S] Competitive inhibitors bind to the enzyme’s active site
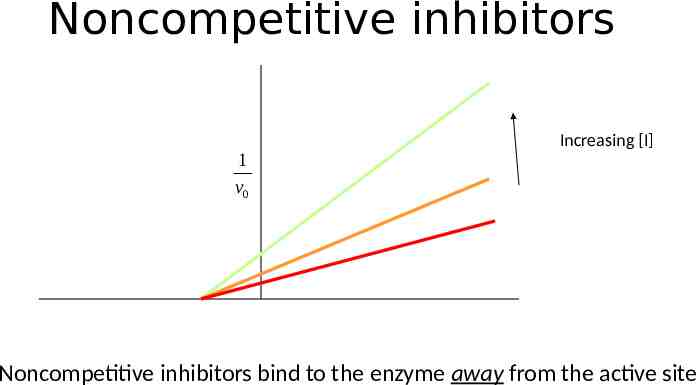
Noncompetitive inhibitors 1 v0 Increasing [I] Noncompetitive inhibitors bind to the enzyme away from the active site
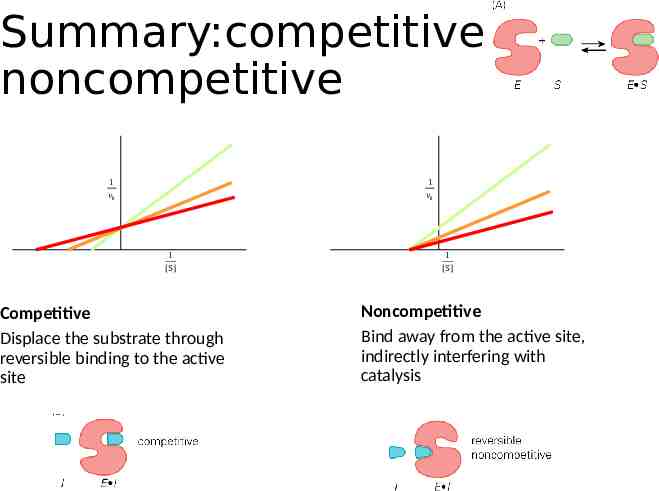
Summary:competitive vs noncompetitive 1 v0 1 v0 1 [S] Competitive Displace the substrate through reversible binding to the active site 1 [S] Noncompetitive Bind away from the active site, indirectly interfering with catalysis
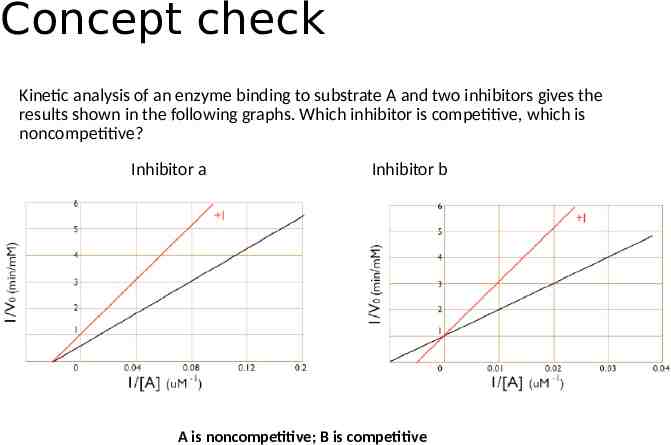
Concept check Kinetic analysis of an enzyme binding to substrate A and two inhibitors gives the results shown in the following graphs. Which inhibitor is competitive, which is noncompetitive? Inhibitor a Inhibitor b A is noncompetitive; B is competitive

Drug cocktails For rapidly evolving diseases (virus, bacteria, cancer), treatment with one drug alone allows for the disease to evolve resistance to the drug Drug cocktails are combinations of several drugs, including multiple drugs that hit the same enzyme but in different ways Harder to evolve resistance to two drugs that bind to different sites of an enzyme simultaneously AND retain function Best drug cocktails combine competitive and noncompetitive inhibitors Given the data provided to you, which drug cocktail do you think will be most effective? Drugs 1 2 Drugs 1 3 Drugs 2 3

Learning goal and objectives Learning Goal: To understand how structural and biochemical data can be used to rationally design drugs. Learning Objectives: Be able to: Explain how we quantitatively describe “structural similarity” Distinguish “competitive” vs “noncompetitive” enzyme inhibition (structurally and biochemically) Use enzymatic kinetic data to determine which inhibitors would combine to make the best drug cocktail
Additional considerations in drug design and testing Toxicity DNA polymerase Drug has to inactivate pathogenic enzymes, but not ours HIV Rev Transcriptase Bioavailabilty Biostabilty Inactive or toxic Economics






