Nuclear Reactions
43 Slides949.50 KB

Nuclear Reactions
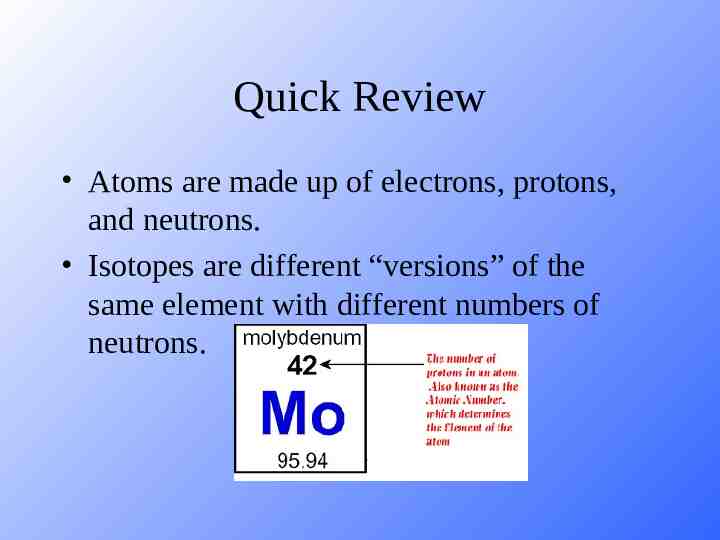
Quick Review Atoms are made up of electrons, protons, and neutrons. Isotopes are different “versions” of the same element with different numbers of neutrons.
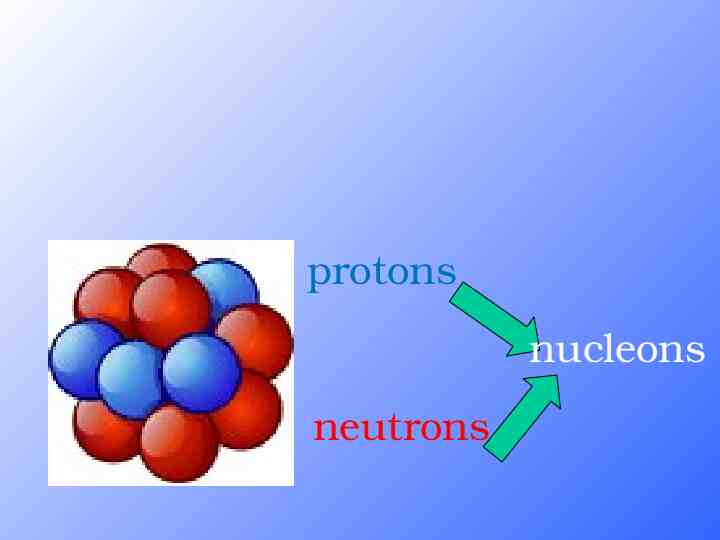
protons nucleons neutrons
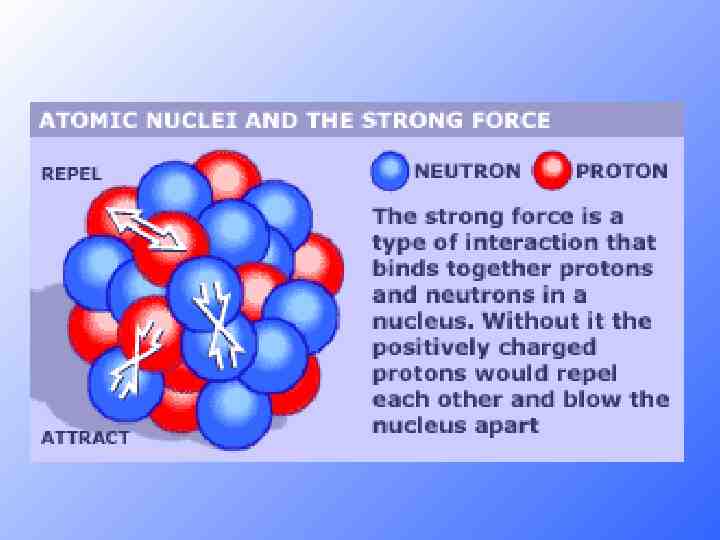
Strong Force Holds the nucleus together, neutrons act like nucleus glue holding it all together. Larger nucueus needs more neutrons to be stable, this limits atom size. Neutrons are not stable on their own and will decay into a proton and electron if left on their own.

Most isotopes naturally are stable. which occur A few naturally occurring isotopes and all of the man-made isotopes are unstable. All elements heavier then Bismuth (atomic number 83)

Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/radio nuclides.

Radioactive Decay Radioactive decay results in the emission of either: an alpha particle ( ), a beta particle ( ), or a gamma ray

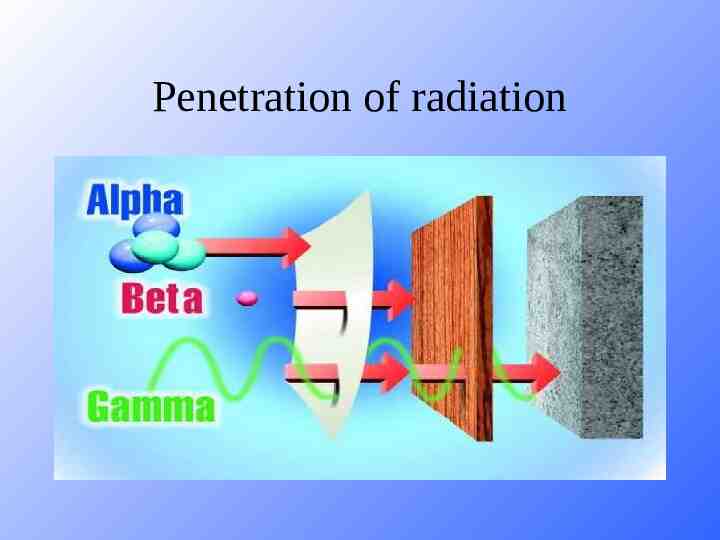
Penetration of radiation
Radioactive decay can also result in: the emission of a positron, or in the capture of an electron.

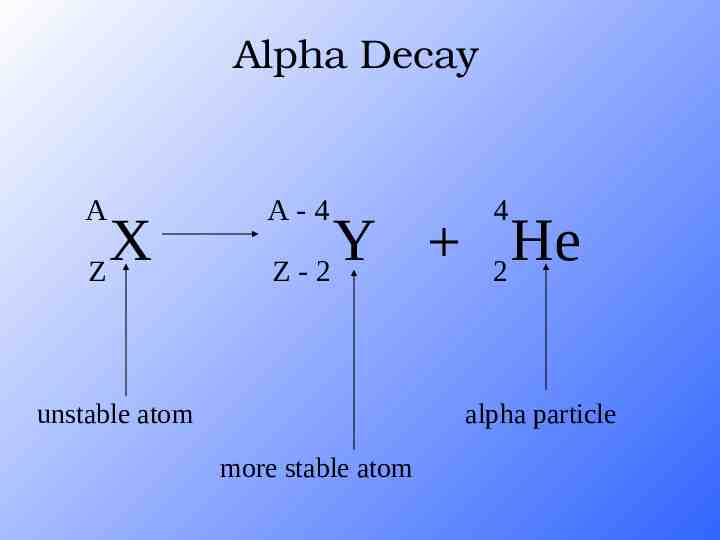
Alpha Decay An alpha particle is identical to that of a helium nucleus. It contains two protons and two neutrons.

Alpha Decay Atomic mass decreases by 4 Atomic # decreases by 2 This type of radiation can be stopped by a piece of paper, it is not very penetrating, BUT if ingested it can be very damaging to body tissues.

Alpha Decay A X Z A-4 4 Y He Z-2 2 unstable atom alpha particle more stable atom
Alpha Decay A A-4 4 226 222 4 X Z Ra 88 Y Z-2 Rn 86 He 2 He 2
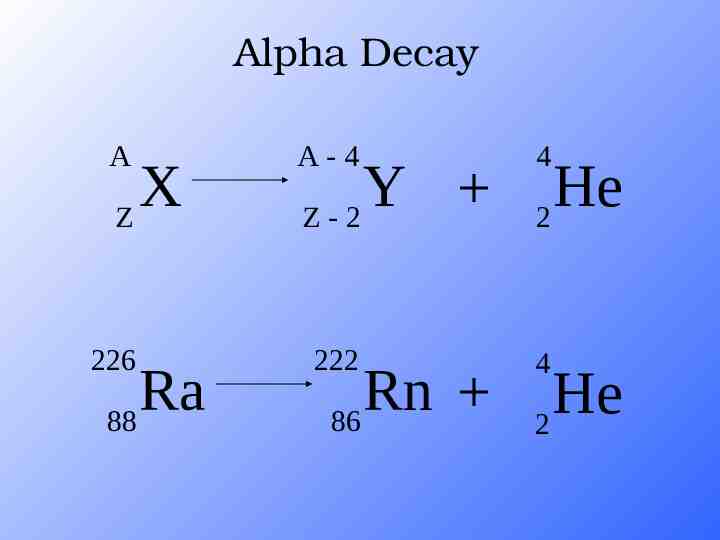
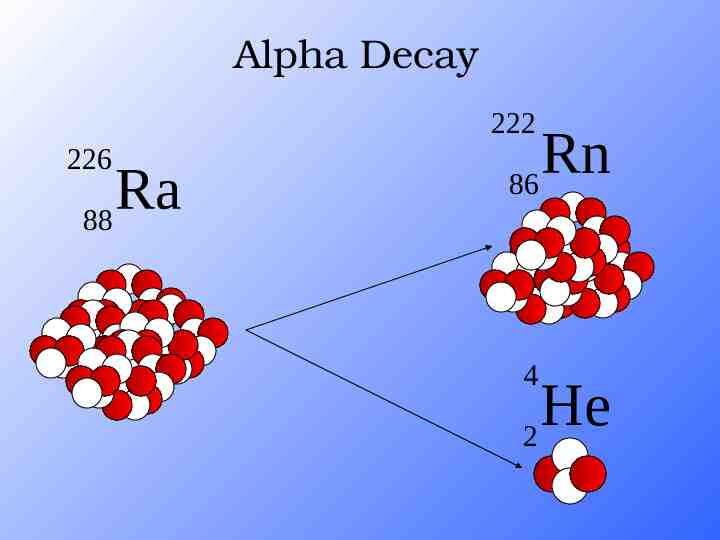
Alpha Decay 222 226 Ra 88 Rn 86 4 He 2
Alpha Decay 222 Rn 86 222 Rn 86 A 4 Y He Z 2 218 Po 84 4 He 2
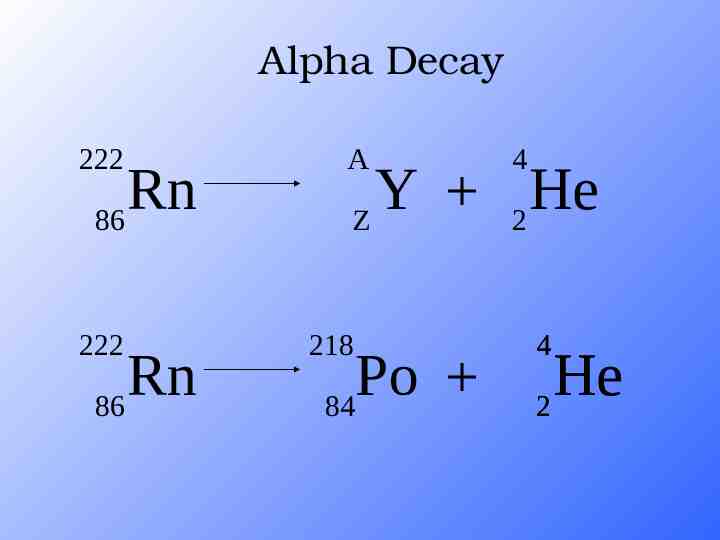
Alpha Decay A 230 4 234 230 4 X Z U 92 Th He 90 2 Th He 90 2
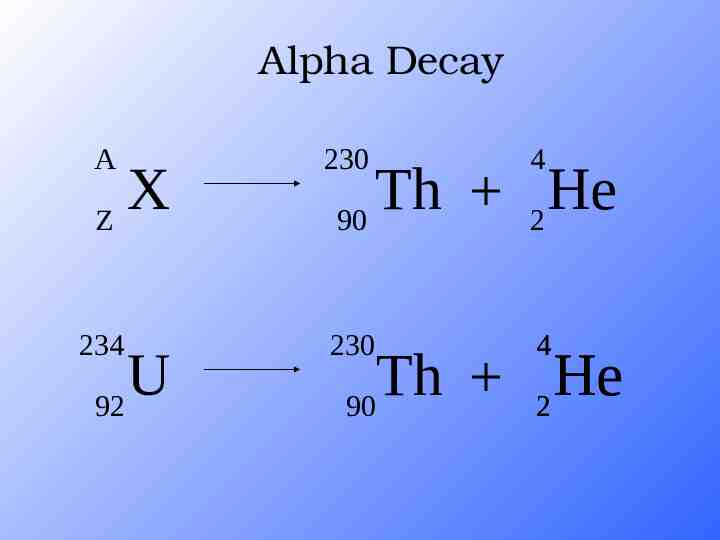
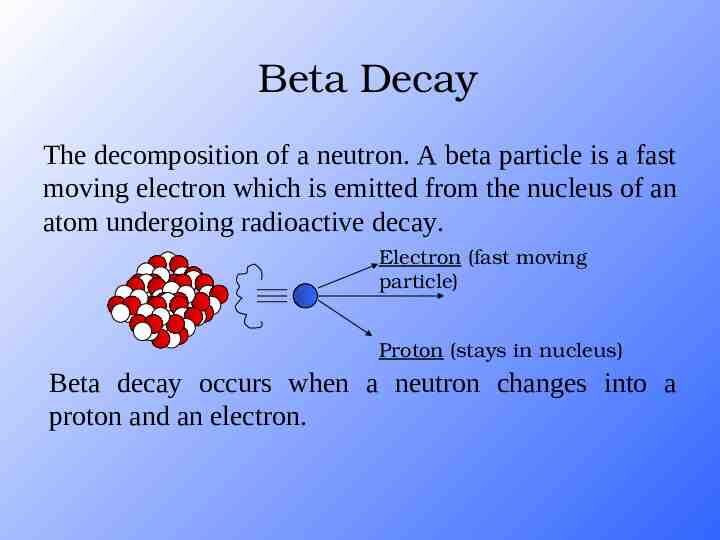
Beta Decay The decomposition of a neutron. A beta particle is a fast moving electron which is emitted from the nucleus of an atom undergoing radioactive decay. Electron (fast moving particle) Proton (stays in nucleus) Beta decay occurs when a neutron changes into a proton and an electron.
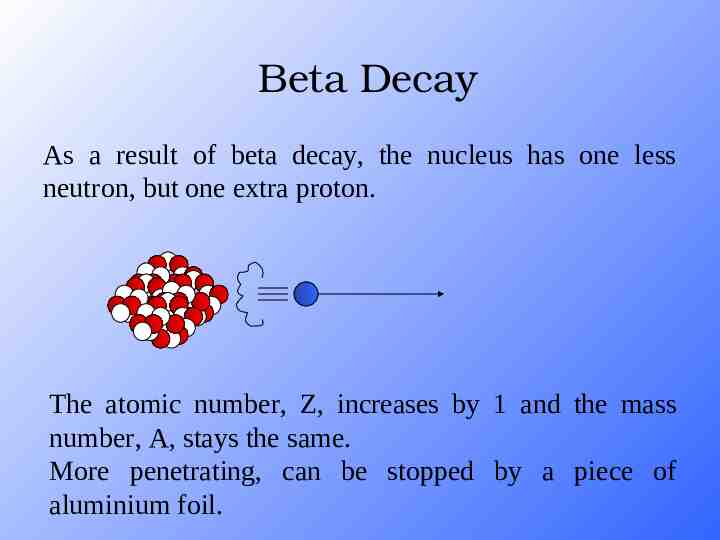
Beta Decay As a result of beta decay, the nucleus has one less neutron, but one extra proton. The atomic number, Z, increases by 1 and the mass number, A, stays the same. More penetrating, can be stopped by a piece of aluminium foil.
Beta Decay 218 218 Po 84 At 85 0 -1
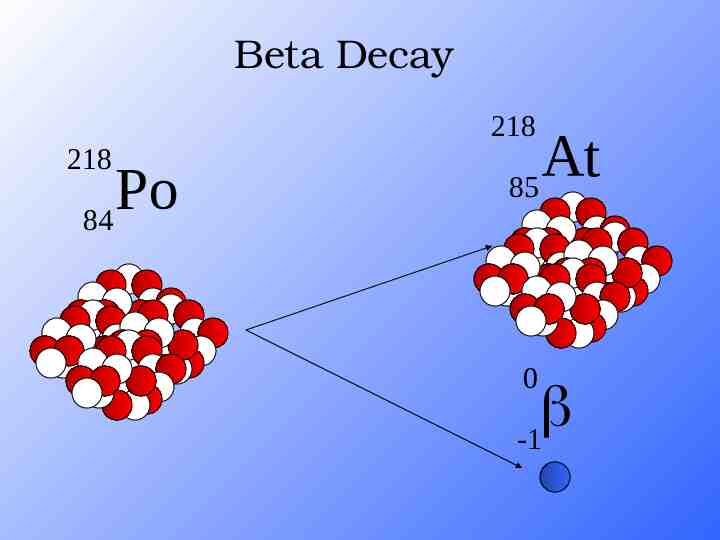
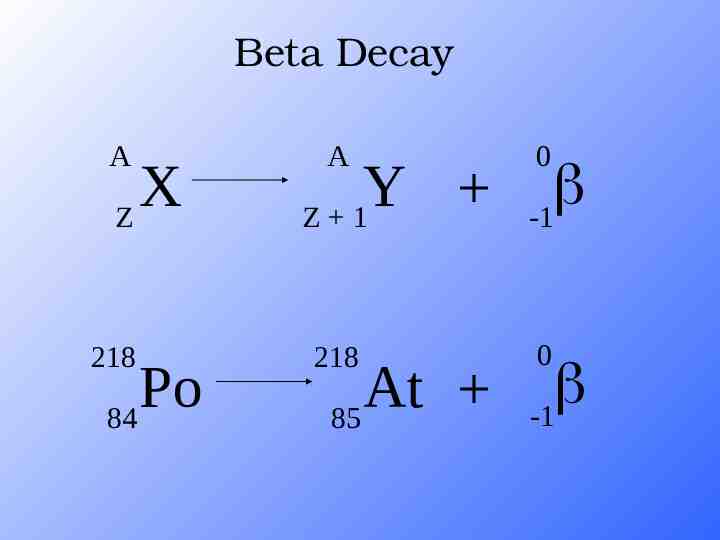
Beta Decay A X Z 218 Po 84 A Y Z 1 218 At 85 0 -1 0 -1
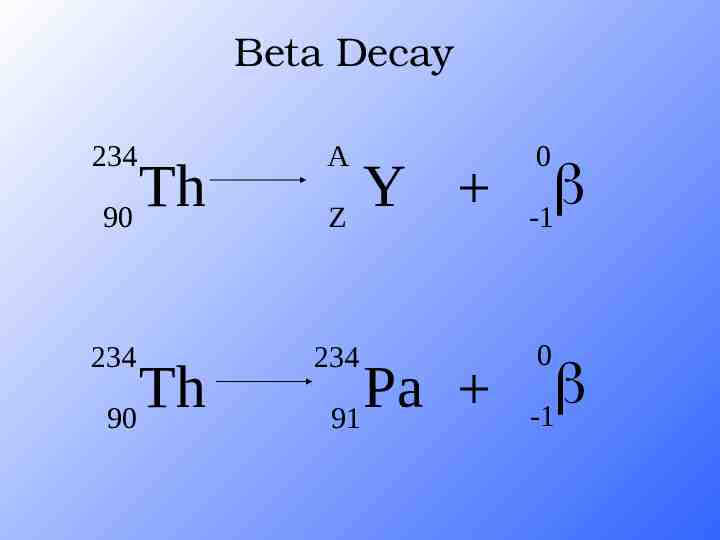
Beta Decay 234 A 234 234 Th 90 Th 90 Y Z Pa 91 0 -1 0 -1
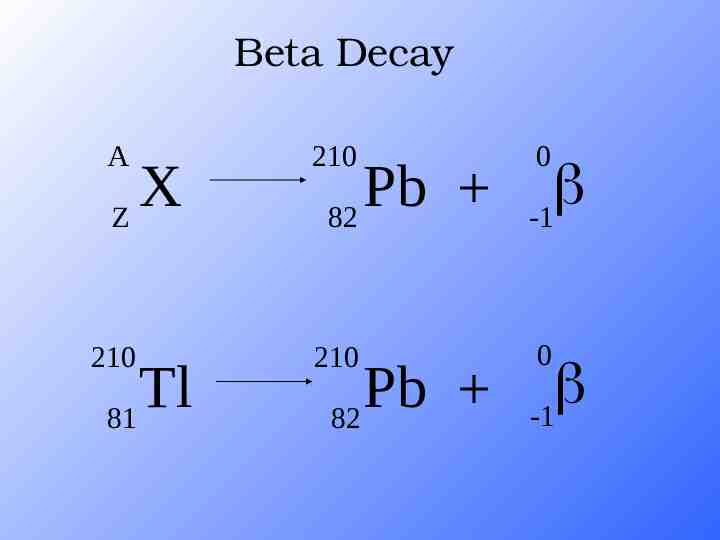
Beta Decay A 210 210 210 X Z Tl 81 Pb 82 Pb 82 0 -1 0 -1
Gamma Decay Gamma rays are not charged particles like and particles. Gamma rays are electromagnetic radiation with high frequency. When atoms decay by emitting or particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely stable. This excess energy is emitted as gamma rays (gamma ray photons have energies of 1 x 10-12 J).
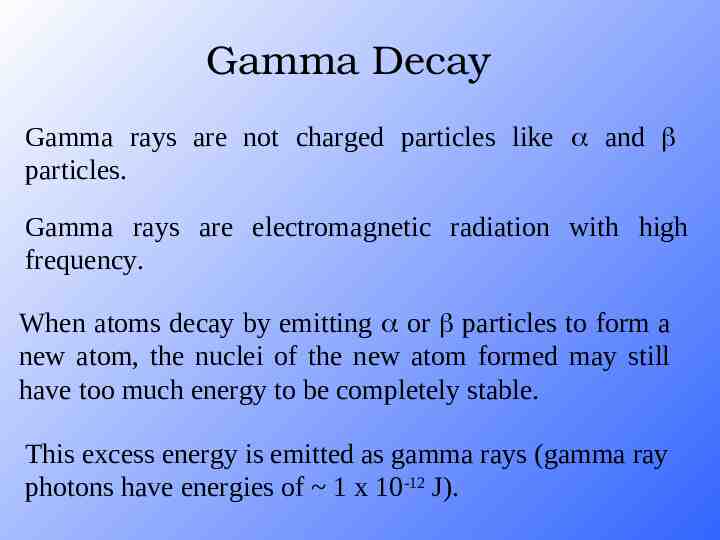
Gamma Radiation Emitted along with alpha and beta radiation. No mass, no charge, no effect on atomic mass or atomic number HIGH penetration, blocked by lead Act like x-rays, but come from a different source.
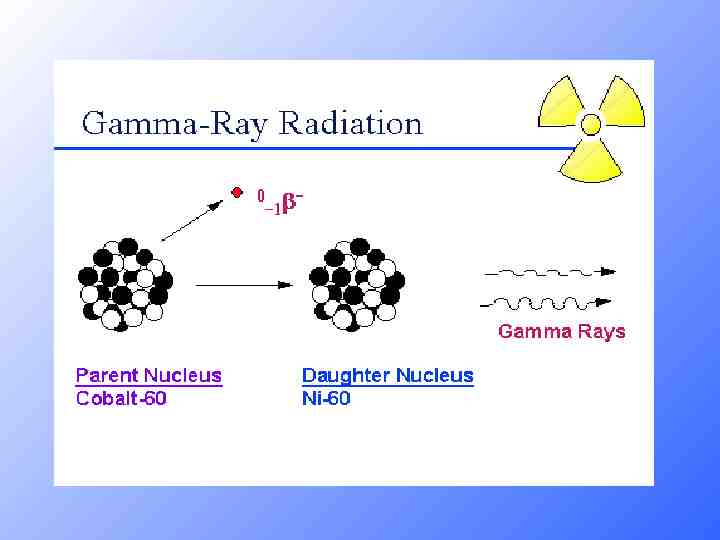
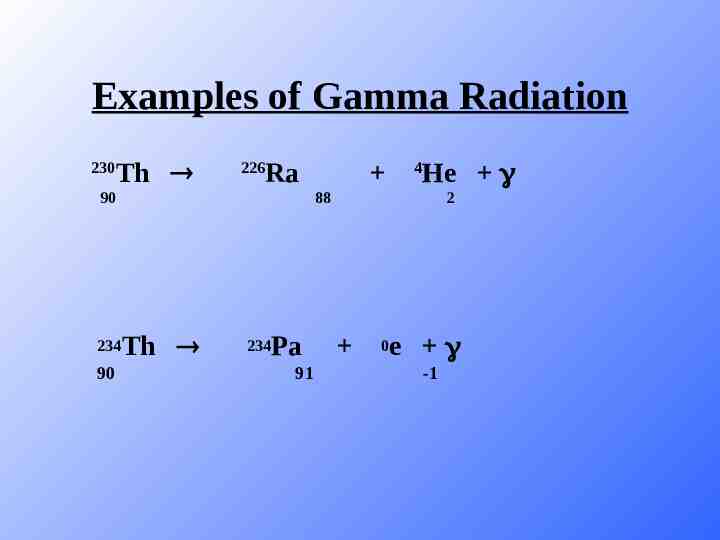
Examples of Gamma Radiation 230 Th 226 Ra 90 234 90 He 4 88 Th 234 Pa 91 2 0 e -1

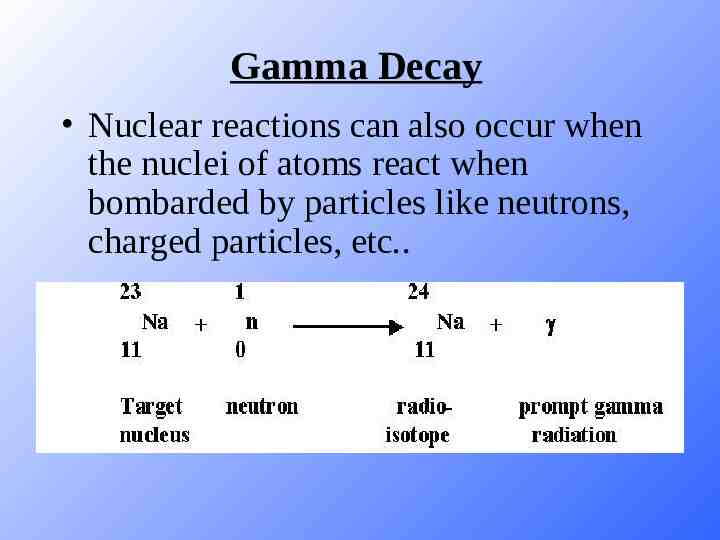
Gamma Decay Nuclear reactions can also occur when the nuclei of atoms react when bombarded by particles like neutrons, charged particles, etc.
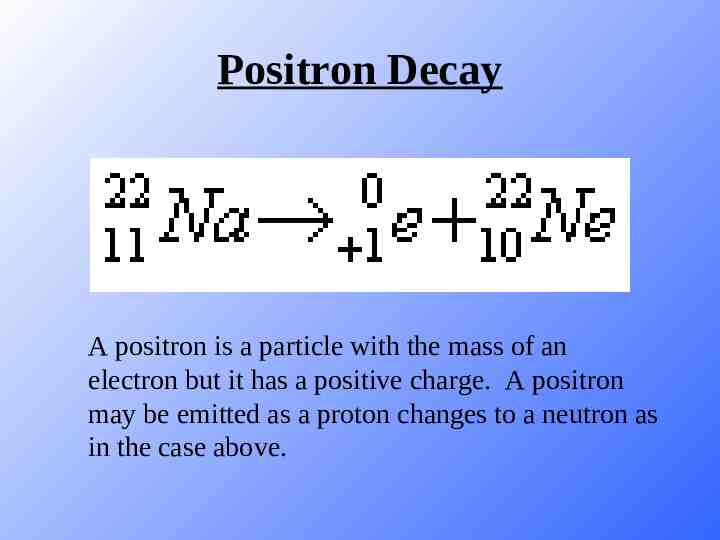
Positron Decay A positron is a particle with the mass of an electron but it has a positive charge. A positron may be emitted as a proton changes to a neutron as in the case above.
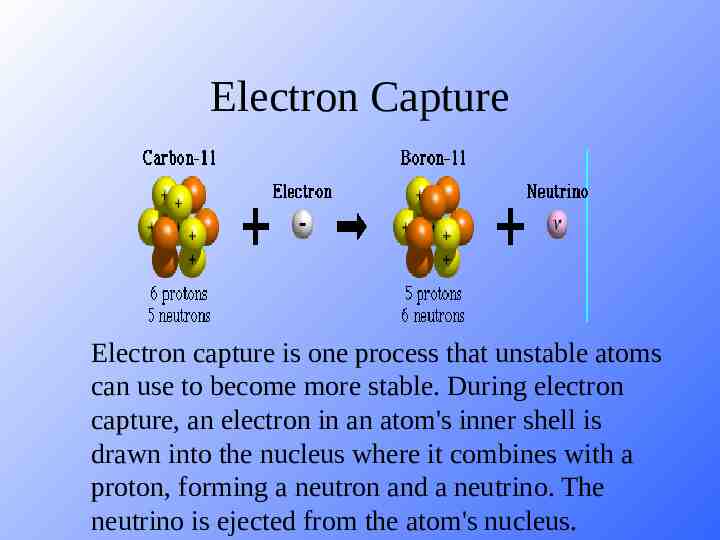
Electron Capture Electron capture is one process that unstable atoms can use to become more stable. During electron capture, an electron in an atom's inner shell is drawn into the nucleus where it combines with a proton, forming a neutron and a neutrino. The neutrino is ejected from the atom's nucleus.
Neutrino Neutrinos are similar to the more familiar electron, with one crucial difference: neutrinos do not carry electric charge.
What do we mean by “unstable”? Nuclear Stability depends on neutron to proton ratios Atomic # 20 wants a ratio of 1 (P N) Atomic # 20 wants more neutrons then protons, with a stable ration being around 1.5 ALL nuclei over 83 are unstable (too big)

How do they become “stable”? Too many neutrons turn N to P(beta decay) Too few neutrons turn P to N (capture an electron and emit a positron)

Half-Life The half-life is the amount of time it takes for half of the atoms in a sample to decay. The half-life for a given isotope is always the same; it doesn't depend on how many atoms you have or on how long they've been sitting around.
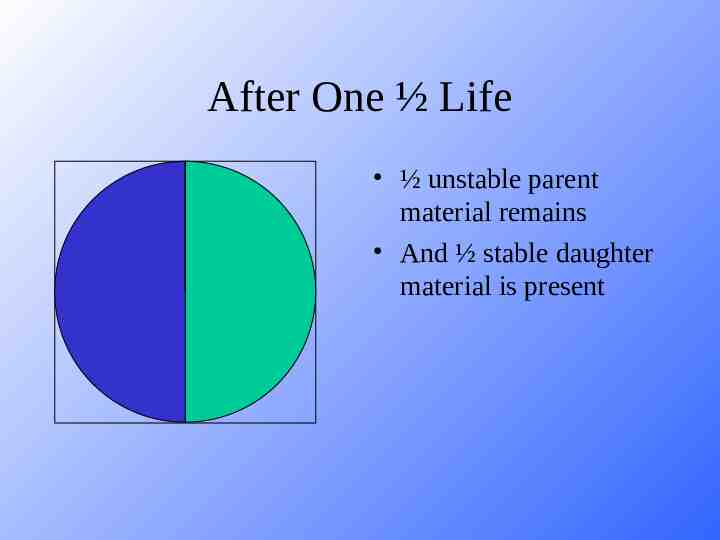
After One ½ Life ½ unstable parent material remains And ½ stable daughter material is present

After Two 1/2 Lives ¼ parent material And ¾ daughter material
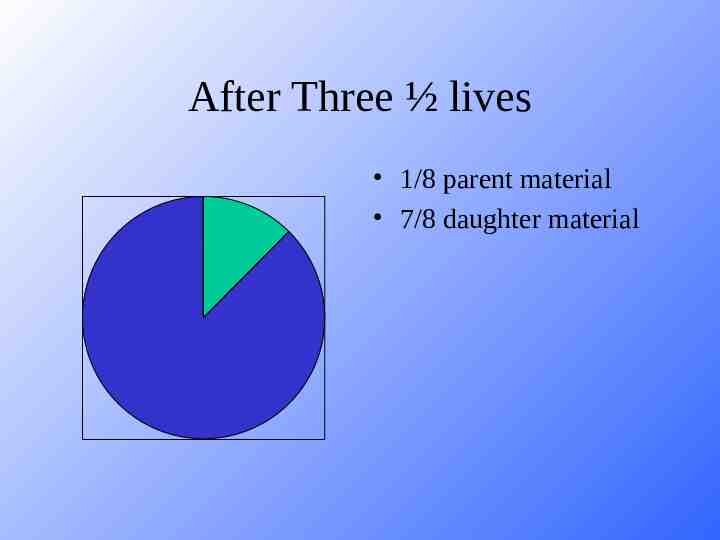
After Three ½ lives 1/8 parent material 7/8 daughter material
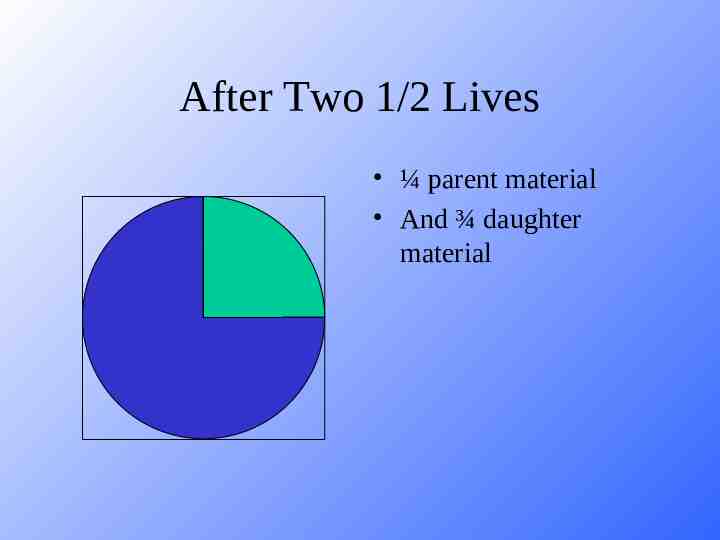
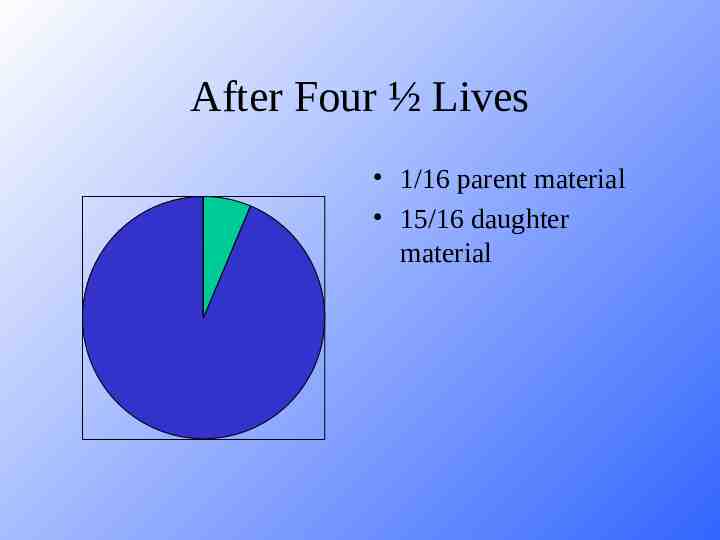
After Four ½ Lives 1/16 parent material 15/16 daughter material
Calculating Half-life A A0 (1/2)n A0 Initial amount of radioactive material A Final amount of radioactive material n number of half-lives
Practice Problem Nitrogen-13 emits beta radiation and decays to carbon-13 with a half-life of 10 minutes. Assume that your starting off with 2 g of the radioisotope nitrogen-13. How many grams of the isotope will still be present after 30 minutes?

Solution A A0 (1/2)n A0 2g n 3 Solve for A A 2 g (1/2)3 A 0.25 g

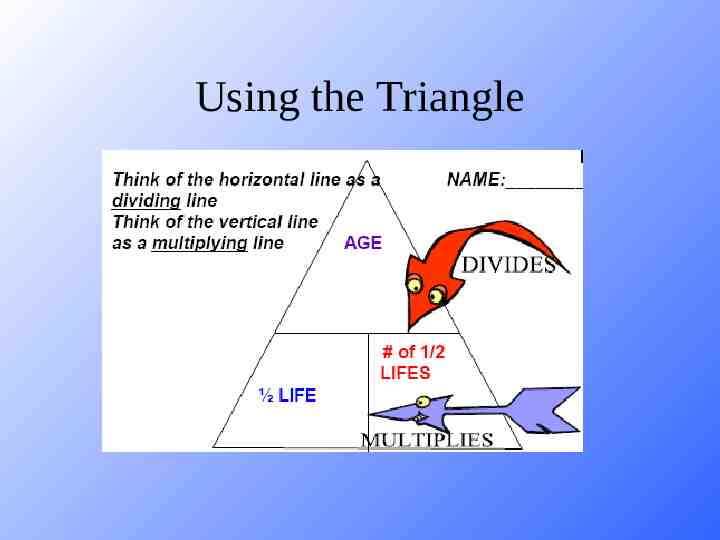
Using the Triangle
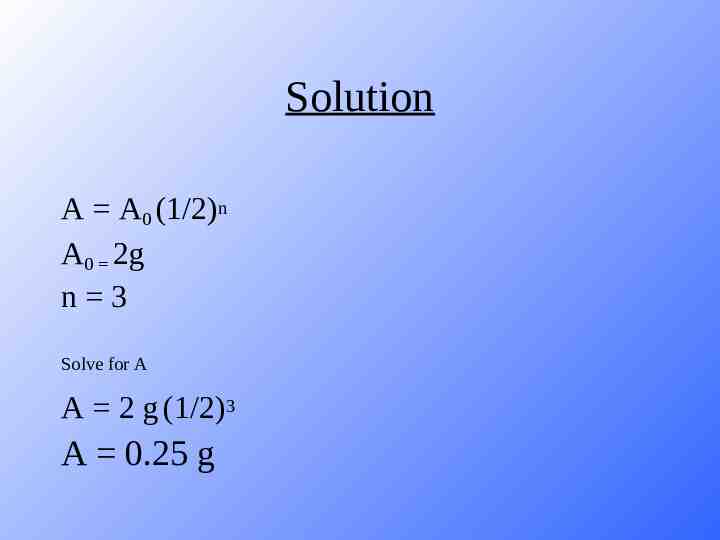
Examples: