Natural Health Products Directorate Initiatives & Updates Stephanie Di
33 Slides927.50 KB

Natural Health Products Directorate Initiatives & Updates Stephanie Di Trapani A/Unit Head, Product Submission Coordination Unit Natural Health Products Directorate Health Canada Presented to the Canadian Association of Professional Regulatory Affairs Toronto, ON – May 2011

Presentation Overview Introduction Quick Facts Application Management Policy Product Licensing Application Strategies Outreach & Engagement Looking Ahead Risk Based Approach – Site Licensing Appendices 2

Acronym Guide AMP – Application Management Policy BEEP - NHPD email list which reaches all NHPD applicants and licensees PAC – Program Advisory Committee PCI – Pre-Cleared Information (monographs, labelling standards, etc) PLA – Product Licence Application form ePLA – Electronic Product Licence Application form UPLAR – Unprocessed Product Licence Applications Regulations 3
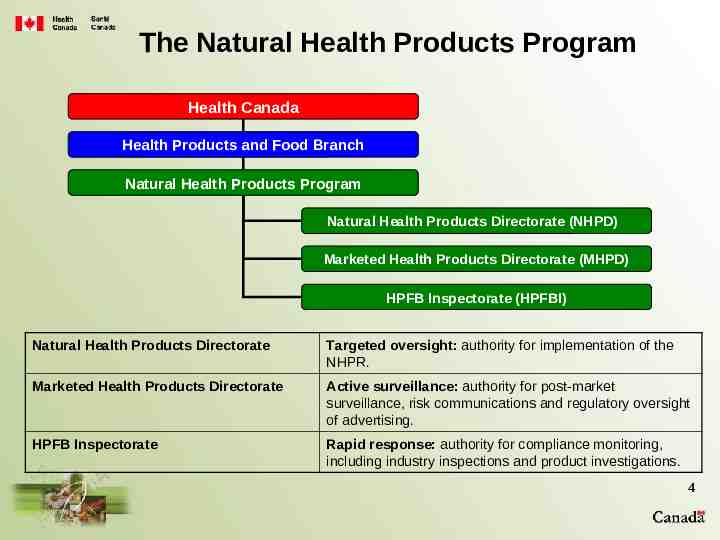
The Natural Health Products Program Health Canada Health Products and Food Branch Natural Health Products Program Natural Health Products Directorate (NHPD) Marketed Health Products Directorate (MHPD) HPFB Inspectorate (HPFBI) Natural Health Products Directorate Targeted oversight: authority for implementation of the NHPR. Marketed Health Products Directorate Active surveillance: authority for post-market surveillance, risk communications and regulatory oversight of advertising. HPFB Inspectorate Rapid response: authority for compliance monitoring, including industry inspections and product investigations. 4
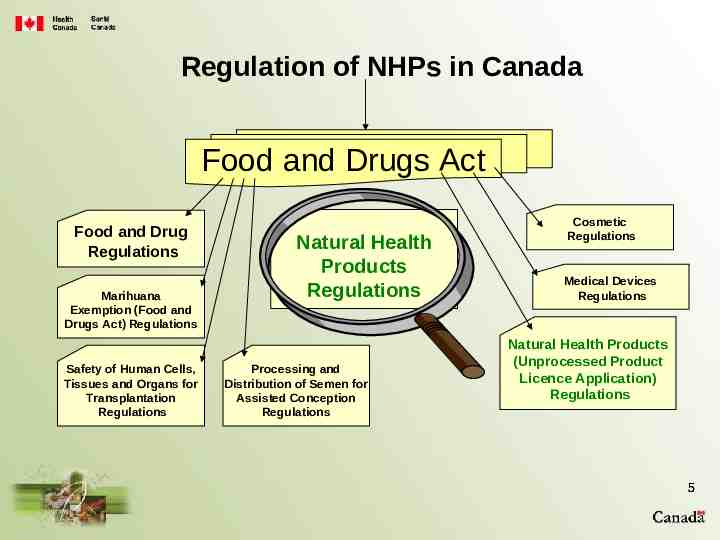
Regulation of NHPs in Canada Food and Drugs Act Food and Drug Regulations Marihuana Exemption (Food and Drugs Act) Regulations Safety of Human Cells, Tissues and Organs for Transplantation Regulations Natural Health Products Regulations Processing and Distribution of Semen for Assisted Conception Regulations Cosmetic Regulations Medical Devices Regulations Natural Health Products (Unprocessed Product Licence Application) Regulations 5

Product Licensing: NHPD Quick Facts Since the implementation of the NHPRs: Health Canada has received over 60,000 Product Licence Applications (PLAs) Over 28,128 PLs representing over 38,509 products, have been issued since 2004. Over 1,432 companies now hold PLs for NHPs. Over 7,785 ENs representing over 9,670 products, have been issued since August 4, 2010 NHPD has now issued 1169 Site Licences Stats valid as of May 11, 2011 6

Policy: Management of Product Licence Applications The policy covers: – General Process Overview – PLA Preparation – On-line Solution – Timeline responsibilities for both NHPD & applicants – Re-Filed Applications – Unsolicited Information – PCI & non-PCI Performance Targets Within non-PCI: Non-Traditional, Traditional, Homeopathic, Non-PCI amendments EN issued after 180 days in assessment 7

Proposed Performance Targets PCI: 60 days Non-PCI: 180 days ( 30 day initial assessment) – 90 days assessment – 30 days correspondence – 45 days review – 15 days decision issuance 8

Strategies: The NHPD’s Online Solution Goal: Automated standards-based business information environment for the exchange, review and management of information supporting the process for the review of NHPD applications throughout the product lifecycle Electronic Product Licence Application (e-PLA) eSubmission Builder Canada Post – Secure Email Licensed NHP Database Finished Product Specification Form NHP Ingredients Database 9
Licensed NHP Database http://webprod.hc-sc.gc.ca/lnhpd-bdpsnh/start-debuter.do?language-langage english 10

Finished Product Specification Form FPS form ensures all quality requirements are provided to NHPD Attestation format that allows applicant to use Pre-Cleared Information Recognized Test Methods and Tolerance Limits Improves/speeds up quality assessment process Reduces number of IRNs and points on IRNs FPS User Guide accompanies form http://www.hc-sc.gc.ca/dhp-mps/prodnatur/legislation/docs/guidemanuel fps-spf-eng.php FPS: http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licenprod/form/form fps-spf-eng.php 11

Natural Health Products Ingredient Database NHPID: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.d o?lang eng Late summer 2010 NHPD communicated that MI/NMIs being submitted in PLAs must match data in the NHPID – MI - proper name, common name, source information – NMI - name, purpose, and source* Version 1.4.2 of the ePLA links to the NHPID Additions/amendments to the NHPID can be made by filling out an NHPID Issue form and emailing it to [email protected] New Additions are featured in the What's New section on the NHPID main page 12

NHP Ingredients Database http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do 13
ePLA Form Electronic “smart” form Linked to the Ingredient database – Single ingredient generated monographs Self validation for completion Label text generator built in 14
ePLA Benefits Once validated all minimum PLA form requirements are met Link to NHPID ensures consistent and validated naming conventions Can be submitted electronically through PosteCS Moves to review stages faster – minimizes processing checks internally at NHPD 15 15

eSubmission Builder - eSB Desktop Program – Easy installation and use Functions with finalized ePLAs Self validating for completion – Ensures all application requirements are satisfied – Specific to each application type Referencing tagging – Intuitively identify reference documents Unique file type – Saves file as .hcs 16

Online Solution in Numbers 199 Trading Partners registered 40% of the total volume of PLA’s rec’d in 2010 were ePLAs 2011 Uptake increasing incrementally approx. 44% (Q1 50%) e-Submission Builder released in February 2011 NHP Ingredients Database has grown to over 12,700 ingredient entries, including 100 approved dosage forms, NMI purposes, and over 400 recognized quality test methods Coming Soon: 5 Instructional Web Videos 17

Strategies: Types of PCI – Safety & Efficacy 173 Single Ingredient Monographs – 154 of these are Generated for use in the ePLA 18 Product Monographs 14 Abbreviated Labelling Stds (AbLS) 6 NHPD Labelling Stds 18 TPD Cat IV Monographs/Labelling Stds Available through the NHPID: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rech ercheReq.do 18

Outreach & Engagement NHP-PAC & Working Groups Standards of Evidence Compliance & Enforcement Weight Loss/Weight Management Product Testing Requirements Outreach activities Information sharing and information gathering Stakeholders Webinars Hands-on workshops Pre-submission meetings Email subscription service Client Service email accounts Access to Submission Coordinators for questions 19

Outreach & Engagement NHPD held a webinar series to increase the usage of the NHP Online System tools: Over 1000 applicants attended these sessions. Since the webinars, NHPD has seen a significant increase in the use of NHP Online tools. NHPD continues to improve and expand the NHP Online toolset and is focusing on stakeholder outreach and education to increase usage Guidance NHPD will be modifying Safety, Efficacy, and Quality guidance documents 20

Looking Ahead - Halted Files Probiotics NHPD has encountered a number of challenges as a result of reviewing evidence submitted for probiotic products in the non-traditional assessment stream. Based on these challenges NHPD recognizes the need for clearer guidance regarding documentation requirements and for the use of the term “probiotic.” Key activities: NHPD anticipates sending out additional communication in the future to clarify the requirements. 21

Looking Ahead - Halted Files Enzymes NHPD has been working on creating assessment criteria as there is limited evidence to support the safety and efficacy of products containing enzyme ingredients. Key activities: NHPD has consulted with various advisory committees in addition to an independent health practitioner a practising naturopathic doctor) to provide advice on the use of enzymes. NHPD has drafted Abbreviated Labelling Standards (AbLS) to support the efficacy and/or safety of the following enzyme ingredients: pancreatin, bromelain, papain, amylase, lipase, cellulase, alpha-galactosidase, trypsin and chymotrypsin and an AbLS to support efficacy for fungal proteases. NHPD will send out communication in the future to clarify criteria. 22

Looking Ahead - Halted Files Energy Drinks Health Canada has become aware of Adverse Reactions reported in relation to energy drink natural health products that raise potential concerns regarding the safe use of these natural health products. Key activities: An Expert Panel on Caffeinated Energy Drinks was created and convened on October 26, 2010. The Expert Panel Report has been finalized and shared with Health Canada. Health Canada is considering the Expert Panel Report and recommendations within the report, along with other information provided to the Department to consider. The composition of the Expert Panel, its Terms of Reference, the Report and Health Canada’s response will be published once completed on Health Canada’s website. 23

Looking Ahead - Halted Files Weight management Standards of evidence for weight loss/management products need to be established to determine how much "weight loss" is needed to make such a claim and what strength of evidence is needed to demonstrate weight loss / management. There is currently a wide variation amongst international regulators on these issues. Key activities Working Group consisting of variety of expert stakeholders has been established to examine the issues and report to NHP-PAC. NHP-PAC will provide recommendations for NHPD DG regarding standards of evidence for weight loss/ weight management. NHPD will consider recommendations and make final decisions NHPD will publish standards of evidence guidance specific to weight loss / management 24

Looking Ahead - NHP Interfaces NHP/Drug (Cosmetic) Three Product Assessment Against Criteria documents posted in Spring 2009 for: -Antiperspirants, -Diaper Rash Products, and -Medicated Skin Care Products Implemented in August 2009 (feedback and suggestions considered) Completion of Product Assessments Against Criteria for next group of product categories at the Cosmetic/Drug (NHP) interface expected in Spring/Summer 2011: -acne therapy, -antidandruff products, and -antiseptic skin cleansers 25

Risk Based Approach to Site Licensing Project Objectives Provide a greater level of assurance that natural health products (NHPs) sold in Canada meet appropriate standards of quality and safety. Design a risk-based site-licensing system for NHPs that provides a wider range of regulatory oversight to the licensing and on-going monitoring of manufacturers and suppliers of NHPs sold in Canada. 26

RBA SL - Background Ensuring the quality and safety of all healthcare products sold in Canada, including NHPs, is a key responsibility of Health Canada. The current system for issuing and maintaining a site license does not provide an appropriate level of assurance as to the quality of NHPs sold in Canada. A product’s safety is directly linked to its quality. 27

RBASL Technical Working Group Mandate To provide technical views and advice to the NHPD to assist in the development of a concept paper. Members selected for their individual scientific and technical knowledge, experience and expertise relevant to the mandate. Representative of cross-section of industry, including company size, activity, product types and geographical locations. 28

RBASL Next Steps February/ March Finalise concept paper based on TWG recommendations for proposed model, standards and information linkages Internal information sessions Spring/Summer Release concept paper seeking comments from stakeholders External information ‘Roadshows’ 29

Questions? Natural Health Products Directorate Health Products and Food Branch Health Canada 2936 Baseline Road, A.L. 3301A Ottawa, Ontario, CANADA K1A 0K9 (Courier: K2H 1B3) NHPD Web Site: www.healthcanada.gc.ca/nhpd E-mail: [email protected] [email protected] 30

APPENDICES 31

NHP Guidance: e-PLA http://www.hc-sc.gc.ca/dhp-mps/pubs/natur/eplaguide-eng.php Version 1.4 released July 2010; As of Nov. 17, 2010: 145 Trading Partners registered; 387 companies have submitted 4592 e-PLAs; 17 webinar sessions delivered to 1,000 applicants. 32

NHP Guidance: Trading Partners for e-PLA http://www.hc-sc.gc.ca/dhp-mps/pubs/natur/trading part commerce-eng.php 33