Management of Surgical Site Infections: Evidence-Based
71 Slides9.33 MB

Management of Surgical Site Infections: Evidence-Based Systematic Literature Review Adopted by the American Academy of Orthopaedic Surgeons (AAOS) Board of Directors June 9, 2018

The American Academy of Orthopaedic Surgeons 2019 Clinical Practice Guideline on the Management of Surgical Site Infections Douglas Lundy, MD; Alexander McLaren, MD; Peter F. Sturm, MD; Sudheer Reddy, MD; Gregory S. Stacy, MD; Gwo-Chin Lee, MD; Hrayr Basmajian, MD; Thomas Fleeter, MD; Paul Anderson, MD; Sandra B. Nelson, MD; Joseph Hsu, MD; Kim Chillag, MD; Carter Cassidy, MD; Douglas Osmon, MD; Eric Hume, MD; Robert Brophy, MD. AAOS Staff: William O. Shaffer, MD; Deborah S. Cummins, PhD; Jayson N. Murray, MA; Mukaram Mohiuddin, MPH; Danielle Schulte, MS; Mary DeMars; Kaitlyn Sevarino, MBA; Anne Woznica, MLIS, AHIP; Peter Shores, MPH 2019 American Academy of Orthopaedic Surgeons

WHAT IS A CLINICAL PRACTICE GUIDELINE? Clinical Practice Guideline A clinical practice guideline is a series of recommendations created to inform clinicians of best practices, based on best available evidence 2019 American Academy of Orthopaedic Surgeons
GOALS AND RATIONALE OF A CLINICAL PRACTICE GUIDELINE Improve treatment based on current best evidence Guides qualified physicians through treatment decisions to improve quality and efficiency of care Identify areas for future research CPG recommendations are not meant to be fixed protocols; patients’ needs, local resources, and clinician independent medical judgement must be considered for any specific procedure or treatment 2019 American Academy of Orthopaedic Surgeons
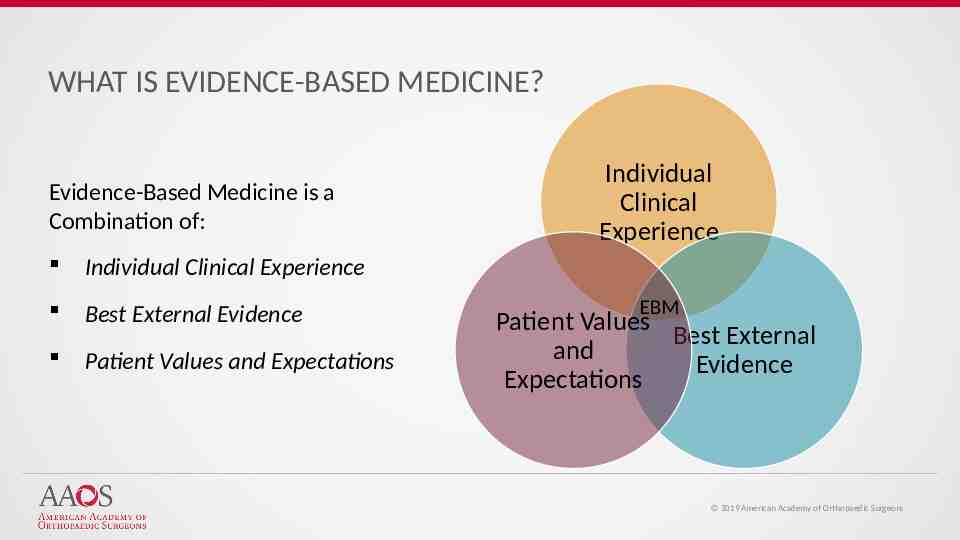
WHAT IS EVIDENCE-BASED MEDICINE? Evidence-Based Medicine is a Combination of: Individual Clinical Experience Best External Evidence Patient Values and Expectations Individual Clinical Experience EBM Patient Values Best External and Evidence Expectations 2019 American Academy of Orthopaedic Surgeons

WHAT IS EVIDENCE-BASED MEDICINE? Evidence-Based Medicine Evidence-based medicine is the conscientious, explicit, and judicious use of current best evidence from clinical care research in the management of individual patients Haynes, Sackett et al, 1996 Transferring evidence from research into practice Sacket et al, 1996, BMJ EBM: what it is and isn’t 2019 American Academy of Orthopaedic Surgeons

IOM STANDARDS FOR DEVELOPING TRUSTWORTHY GUIDELINES Establish Transparency Management of Conflict of Interest Guideline Development Group Composition Clinical Practice Guideline-Systematic Review Intersection Establish Evidence of Foundations for and Rating Strength of Recommendations Articulation of Recommendations External Review Updating 2019 American Academy of Orthopaedic Surgeons
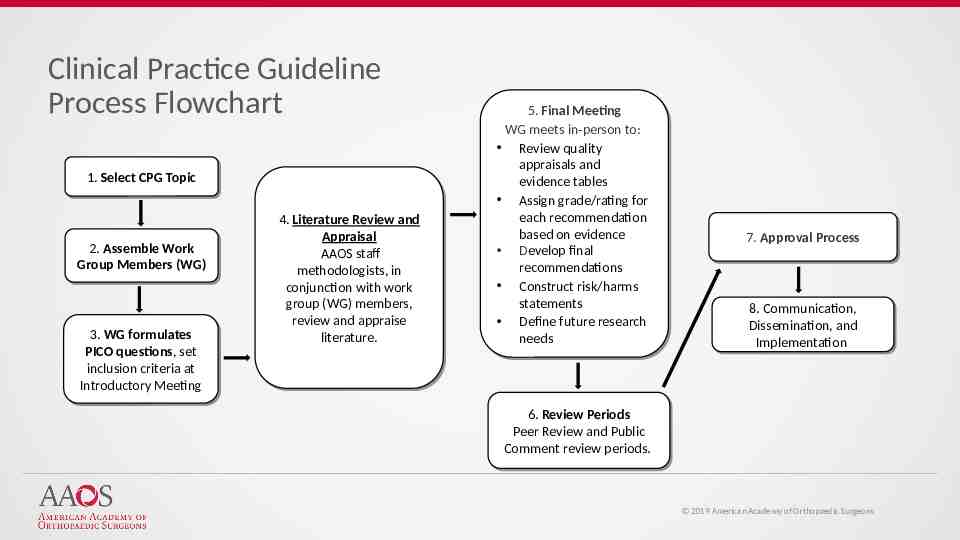
Clinical Practice Guideline Process Flowchart 1. 1. Select Select CPG CPG Topic Topic 2. 2. Assemble Assemble Work Work Group Group Members Members (WG) (WG) 3. 3. WG WG formulates formulates PICO PICO questions, questions, set set inclusion inclusion criteria criteria at at Introductory Introductory Meeting Meeting 4. 4. Literature Literature Review Review and and Appraisal Appraisal AAOS AAOS staff staff methodologists, methodologists, in in conjunction with work conjunction with work group group (WG) (WG) members, members, review review and and appraise appraise literature. literature. 5. 5. Final Final Meeting Meeting WG WG meets meets in-person in-person to: to: Review Review quality quality appraisals appraisals and and evidence evidence tables tables Assign Assign grade/rating grade/rating for for each each recommendation recommendation based based on on evidence evidence Develop final Develop final recommendations recommendations Construct Construct risk/harms risk/harms statements statements Define Define future future research research needs needs 7. 7. Approval Approval Process Process 8. 8. Communication, Communication, Dissemination, Dissemination, and and Implementation Implementation 6. 6. Review Review Periods Periods Peer Review and Peer Review and Public Public Comment Comment review review periods. periods. 2019 American Academy of Orthopaedic Surgeons
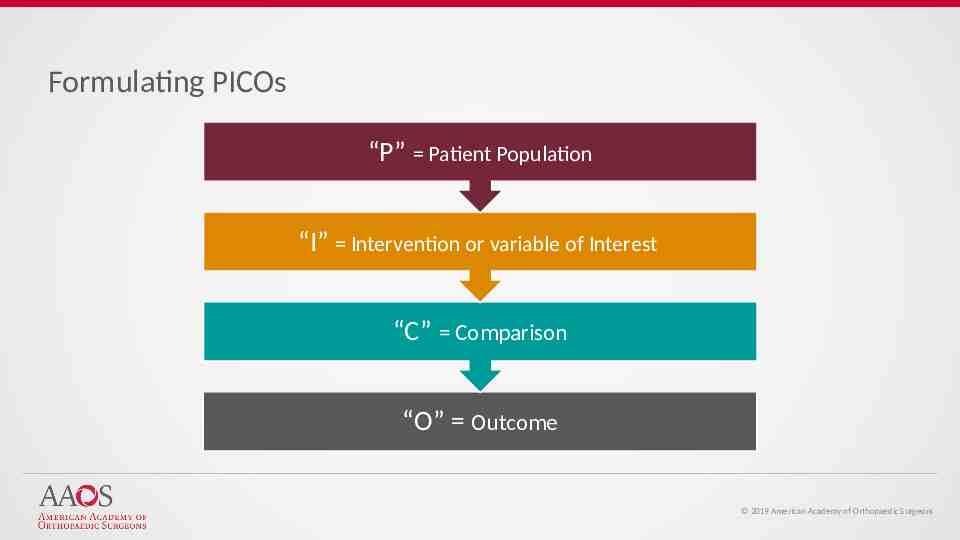
Formulating PICOs “P” Patient Population “I” Intervention or variable of Interest “C” Comparison “O” Outcome 2019 American Academy of Orthopaedic Surgeons

Inclusion/Exclusion Criteria Standard inclusion criteria include: Must study humans Must be published in English Must be published in or after 1966 Can not be performed on cadavers Work group members define additional exclusion criteria based on PICO question 2019 American Academy of Orthopaedic Surgeons

Literature Searches Databases used: PubMed EMBASE (Excerpta Medica dataBASE) CINAHL (Cumulative Index of Nursing and Allies Health Literature) Cochrane Central Register of Controlled Trials Search using key terms from work group’s PICO questions and inclusion criteria Secondary manual search of the bibliographies of all retrieved publications for relevant citations Recalled articles evaluated for inclusion based on the study selection criteria 2019 American Academy of Orthopaedic Surgeons

Best Evidence Synthesis Include only highest quality evidence for any given outcome if available If there are fewer than two occurrences of an outcome of this quality, the next lowest quality is considered until at least two occurrences have been acquired. 2019 American Academy of Orthopaedic Surgeons

STRENGTH OF RECOMMENDATIONS STRENGTH OVERALL STRENGTH OF EVIDENCE STRONG Two or more HIGH Strength Studies with consistent findings MODERATE 1 HIGH OR 2 MODERATE strength studies with consistent findings LIMITED One or more LOW strength studies and/or only 1 MODERATE strength study with consistent findings or evidence from a single, or the evidence is insufficient, or conflicting CONSENSUS Expert opinion (no studies) No supporting evidence in the absence of reliable evidence. Work group is making a recommendation based on their clinical opinion STRENGTH VISUAL 2019 American Academy of Orthopaedic Surgeons
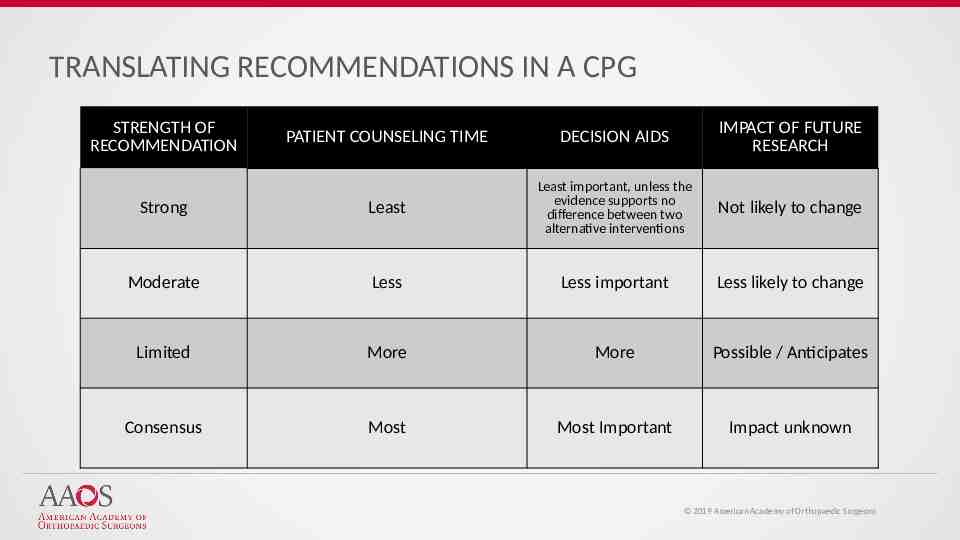
TRANSLATING RECOMMENDATIONS IN A CPG STRENGTH OF RECOMMENDATION PATIENT COUNSELING TIME DECISION AIDS IMPACT OF FUTURE RESEARCH Strong Least Least important, unless the evidence supports no difference between two alternative interventions Not likely to change Moderate Less Less important Less likely to change Limited More More Possible / Anticipates Consensus Most Most Important Impact unknown 2019 American Academy of Orthopaedic Surgeons

Assessing Quality of Evidence All included studies undergo a quality assessment. Each study’s design is evaluated for risk of bias and receives a final quality grade, depending on the number of study design flaws. Study quality tables are made available to the work group in the final data report and the final publication of the guideline/SR 2019 American Academy of Orthopaedic Surgeons
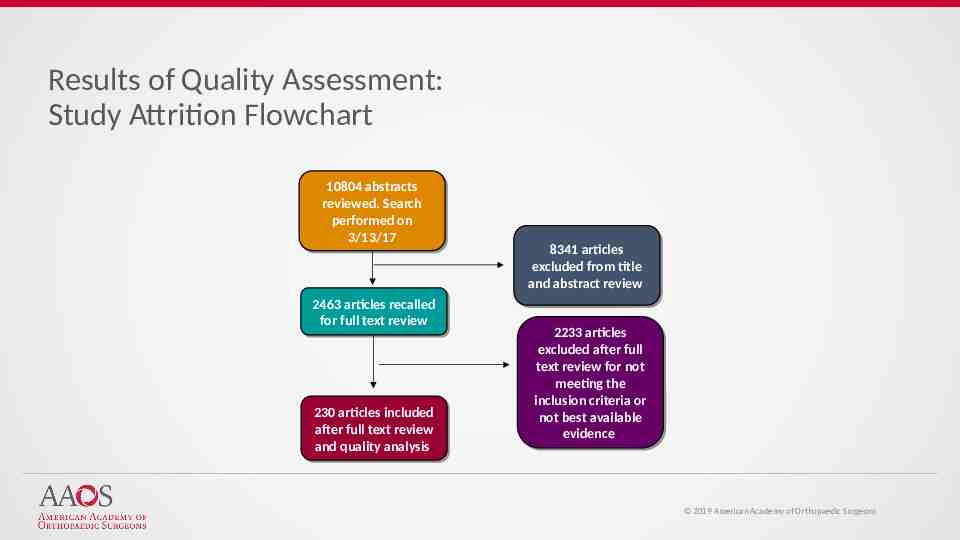
Results of Quality Assessment: Study Attrition Flowchart 10804 10804 abstracts abstracts reviewed. reviewed. Search Search performed performed on on 3/13/17 3/13/17 2463 2463 articles articles recalled recalled for for full full text text review review 230 230 articles articles included included after after full full text text review review and and quality quality analysis analysis 8341 8341 articles articles excluded excluded from from title title and abstract review and abstract review 2233 2233 articles articles excluded excluded after after full full text review for not text review for not meeting meeting the the inclusion inclusion criteria criteria or or not not best best available available evidence evidence 2019 American Academy of Orthopaedic Surgeons

Voting on the Recommendations Recommendations and recommendation strengths voted on by work group during final meeting Approved and adopted by simple majority (60%) when voting on every recommendation If disagreement, further discussion to whether the disagreement could be resolved 2019 American Academy of Orthopaedic Surgeons
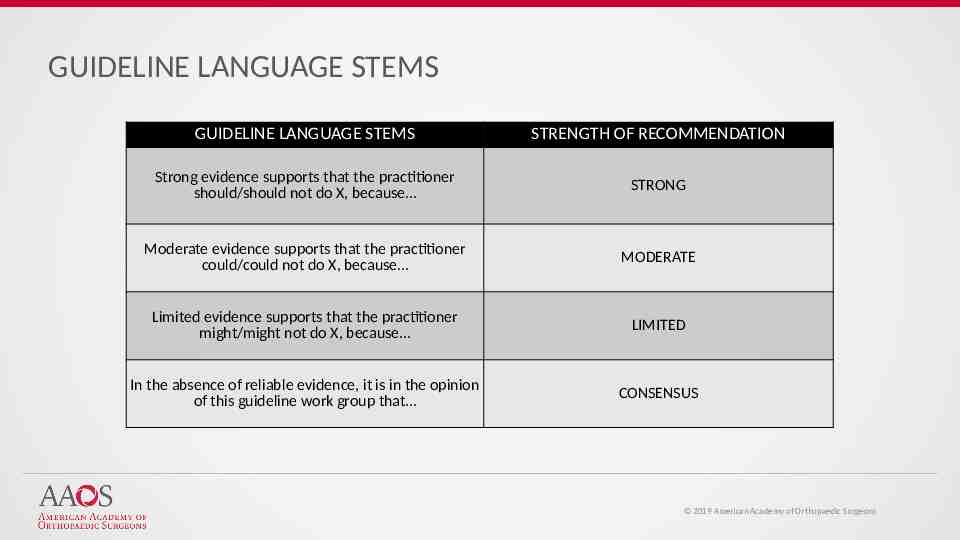
GUIDELINE LANGUAGE STEMS GUIDELINE LANGUAGE STEMS STRENGTH OF RECOMMENDATION Strong evidence supports that the practitioner should/should not do X, because STRONG Moderate evidence supports that the practitioner could/could not do X, because MODERATE Limited evidence supports that the practitioner might/might not do X, because LIMITED In the absence of reliable evidence, it is in the opinion of this guideline work group that CONSENSUS 2019 American Academy of Orthopaedic Surgeons

Peer Review Guideline draft sent for peer review to external experts. Comments and draft of responses reviewed by work group members Recommendation changes required a majority vote by work group A detailed report of all resulting revisions is published with the guideline document 2019 American Academy of Orthopaedic Surgeons

PUBLIC COMMENT Following peer review modifications, CPG undergoes public commentary period Comments are solicited from: AAOS Board of Directors AAOS Council on Research and Quality AAOS Committee on Evidence-Based Quality and Value AAOS Board of Councilors AAOS Board of Specialty Societies 200 commentators have the opportunity to provide input 2019 American Academy of Orthopaedic Surgeons

FINAL MEETING The work group is charged with: Review of data summaries Final recommendation language Rationale and risk/harm construction Future research 2019 American Academy of Orthopaedic Surgeons

Management of Surgical Site Infections Systematic Literature Review Overview Based on a systematic review of published studies Addresses the management of surgical site infections occurring in patients who have undergone orthopaedic surgery Highlights limitations in literature and areas requiring future research Trained physicians and surgeons are intended users 2019 American Academy of Orthopaedic Surgeons
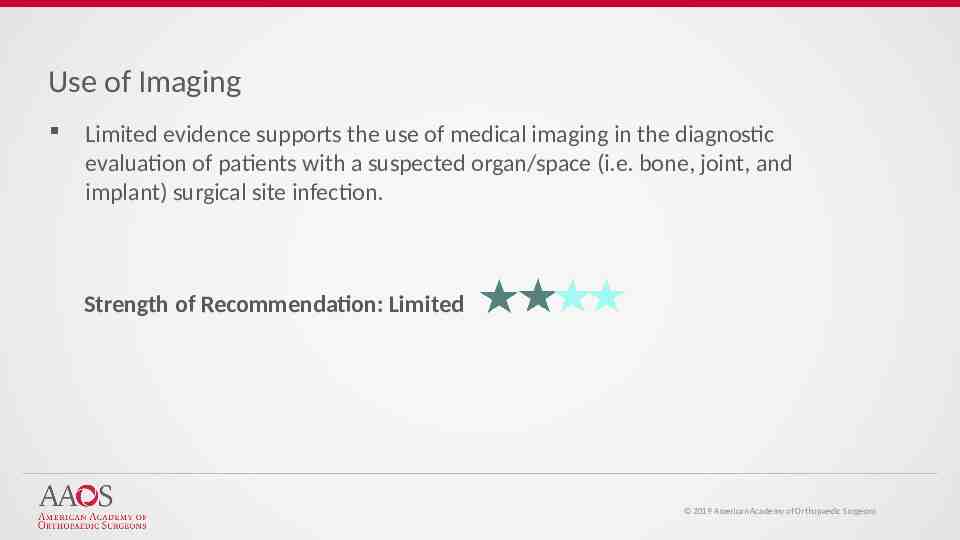
Use of Imaging Limited evidence supports the use of medical imaging in the diagnostic evaluation of patients with a suspected organ/space (i.e. bone, joint, and implant) surgical site infection. Strength of Recommendation: Limited 2019 American Academy of Orthopaedic Surgeons
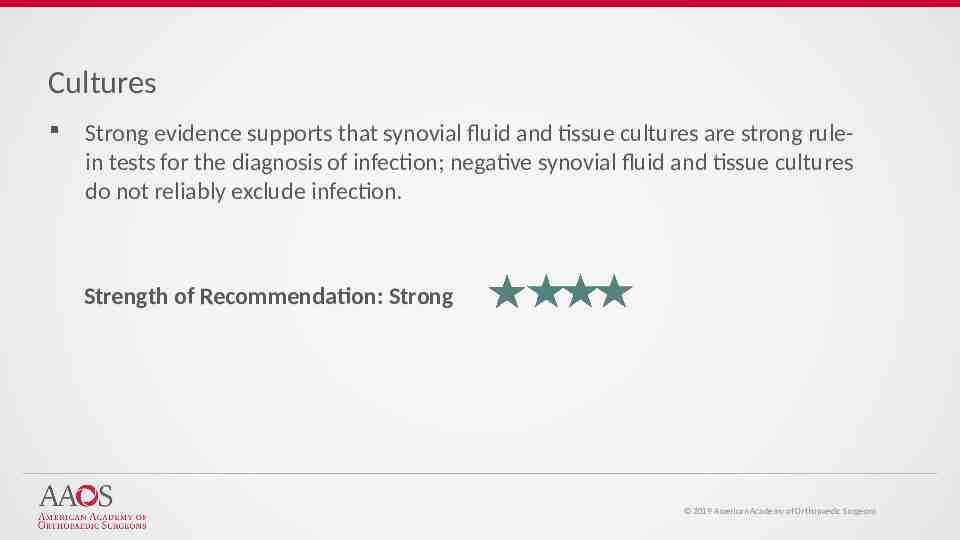
Cultures Strong evidence supports that synovial fluid and tissue cultures are strong rulein tests for the diagnosis of infection; negative synovial fluid and tissue cultures do not reliably exclude infection. Strength of Recommendation: Strong 2019 American Academy of Orthopaedic Surgeons

C-Reactive Protein Strong evidence supports that C-reactive Protein is a strong rule-in and rule-out marker for patients with suspected surgical site infections Strength of Recommendation: Strong 2019 American Academy of Orthopaedic Surgeons

Erythrocyte Sedimentation Rate Limited strength evidence does not support the use of ESR, alone, to rule in and rule out surgical site infections due to conflicting data Strength of Recommendation: Limited 2019 American Academy of Orthopaedic Surgeons
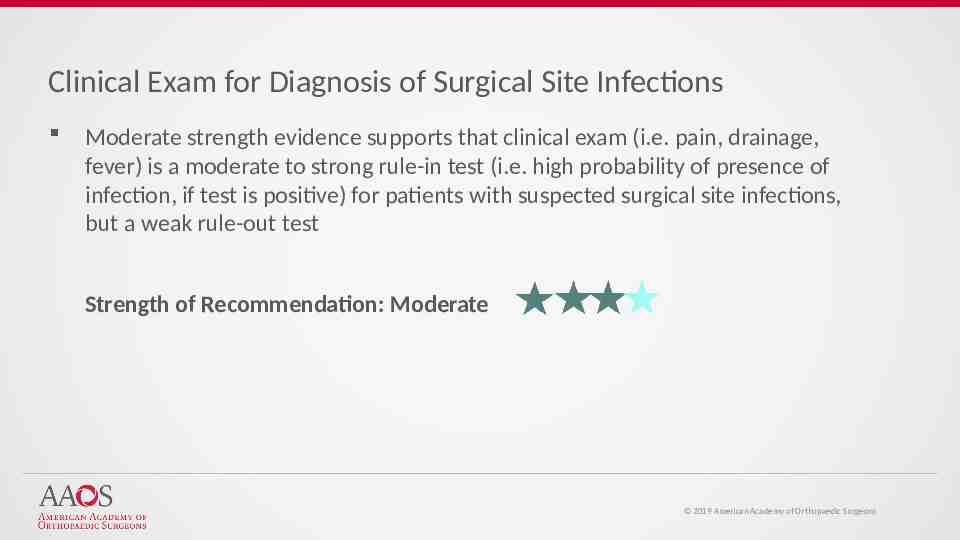
Clinical Exam for Diagnosis of Surgical Site Infections Moderate strength evidence supports that clinical exam (i.e. pain, drainage, fever) is a moderate to strong rule-in test (i.e. high probability of presence of infection, if test is positive) for patients with suspected surgical site infections, but a weak rule-out test Strength of Recommendation: Moderate 2019 American Academy of Orthopaedic Surgeons

Strong Evidence of Factors Associated with Increased Risk of SSI Strong evidence supports that the following factors are associated with an increased risk of infection: Anemia Duration of Hospital Stay Immunosuppressive Medications History of Alcohol Abuse Obesity Depression History of Congestive Heart Failure Dementia HIV/AIDS Strength of Recommendation: Strong 2019 American Academy of Orthopaedic Surgeons

Increased Associated Risk of SSI Moderate strength evidence supports that patients meeting one or more of the following criteria are at an increased risk of infection after hip and knee arthroplasty: Chronic Kidney Disease Diabetes (conflicting evidence) Tobacco Use/Smoking (conflicting evidence) Malnutrition (conflicting evidence) Strength of Recommendation: Moderate 2019 American Academy of Orthopaedic Surgeons

Limited Evidence of Increased Associated SSI Risk Limited strength evidence supports that patients meeting one or more of the following criteria are at an increased risk of infection after hip and knee arthroplasty: Cancer Hypertension (conflicting evidence) Liver Disease (conflicting evidence) Strength of Recommendation: Limited 2018 American Academy of Orthopaedic Surgeons
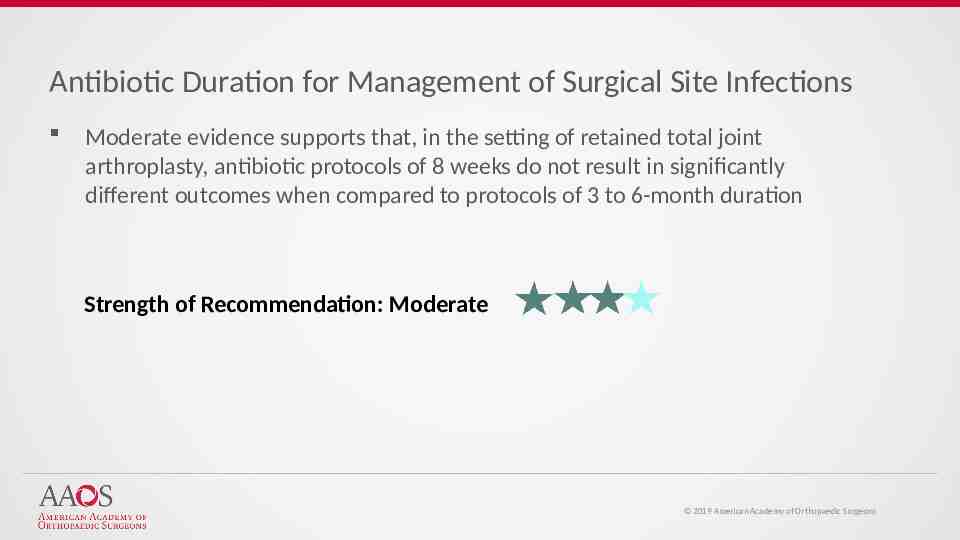
Antibiotic Duration for Management of Surgical Site Infections Moderate evidence supports that, in the setting of retained total joint arthroplasty, antibiotic protocols of 8 weeks do not result in significantly different outcomes when compared to protocols of 3 to 6-month duration Strength of Recommendation: Moderate 2019 American Academy of Orthopaedic Surgeons
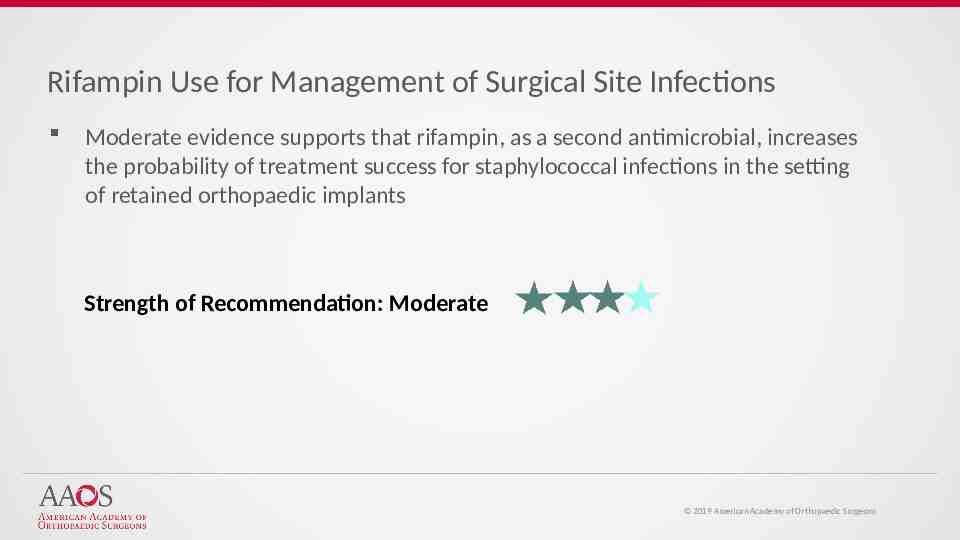
Rifampin Use for Management of Surgical Site Infections Moderate evidence supports that rifampin, as a second antimicrobial, increases the probability of treatment success for staphylococcal infections in the setting of retained orthopaedic implants Strength of Recommendation: Moderate 2019 American Academy of Orthopaedic Surgeons
Adjunctive Treatment In the absence of reliable evidence, it is the opinion of the work group that adjunctive treatment is of limited value in the management of surgical site infections Strength of Recommendation: Consensus 2019 American Academy of Orthopaedic Surgeons
Surgical Timing and Percutaneous Drainage In the absence of reliable evidence, it is the opinion of the work group that the definitive strategy to successfully treat surgical site infections is thorough debridement Strength of Recommendation: Consensus 2019 American Academy of Orthopaedic Surgeons

Surgical Timing In the absence of reliable evidence, it is the opinion of the work group that irrigation and debridement are the cornerstones of successful management of surgical site infections and timely management is crucial, especially in the setting of orthopaedic implants Strength of Recommendation: Consensus 2019 American Academy of Orthopaedic Surgeons

Future Research – Medical Imaging Most of the literature exploring the imaging of suspected postoperative infections pertains to patients with prosthetic joints, with cohorts of patients whose imaging examinations occurred months to years following surgery. Furthermore, there is a lack of data regarding the sensitivity and specificity of imaging tests for the diagnosis of infections during the first 90 days following surgery as well as surgical site infections not associated with implants. Future research exploring the diagnostic value of imaging for surgical site infections in patients with or without orthopaedic implants in the early ( 90 days) postoperative period is necessary. This could include comparative studies between various imaging modalities which may further clarify the utility of each modality for the diagnosis of suspected surgical site infection. 2019 American Academy of Orthopaedic Surgeons

Future Research – Cultures The majority of studies on the role of culture in the diagnosis of surgical site infection stemmed from studies in periprosthetic infection. Development of optimal culture protocols for surgical site infections other than periprosthetic joint infections are needed. Future research directions may also include advanced non-culture based diagnostic modalities including PCR and next generation sequencing. 2019 American Academy of Orthopaedic Surgeons

Future Research – C-Reactive Protein Much of the work on inflammatory markers has been focused upon total joint arthroplasty. Future research should focus on identifying more accurate inflammatory markers, and distinguishing a standardized set of criteria and thresholds to aid in the diagnosis of surgical site infection not only as it pertains to PJI but in other cases of SSI. 2019 American Academy of Orthopaedic Surgeons

Future Research – Erythrocyte Sedimentation Rate ESR is of limited utility in the diagnosis of SSI as an isolated test. Future investigations will likely examine the use of ESR in combination with other diagnostic markers. 2019 American Academy of Orthopaedic Surgeons

Future Research – Clinical Exam for the Diagnosis of an SSI Clinical factors that can be determined from history and physical exam that identify patients at risk for surgical site need further investigation. The possible linkage of persistent fevers and the wound drainage to surgical site infections are needed. Characterization and development of protocols to manage early poorly healing or inflamed wounds are needed. 2019 American Academy of Orthopaedic Surgeons

Future Research Factors Associated with an Increased Risk of Infection Attempts at identifying the optimal length of stay should be continued. Identify optimal discharge pathways for each individual patient. The correlation with early discharge and rates of readmission needs to be assessed. Understand the relative contribution of comorbidity-severity related to the duration of hospital stay. The list of immunosuppressive drugs is expanding rapidly and the research on the effects of these newer drugs is needed. Also, additional information about dosing, discontinuing medication before surgery and additional orthopaedic procedures that might be impacted are also needed. Future trials should examine optimal time for discontinuing immunosuppressive medications prior to surgery. 2019 American Academy of Orthopaedic Surgeons

Future Research Factors Associated with an Increased Risk of Infection Further research is needed on assessment tools to assess the relation of alcohol consumption and surgical risk. Future research is needed to assess the role of nutrition in the modification of obesity and the effects of high BMI and BMI-associated comorbidities on the risk surgical site infections. Needed to see if treatment of depression alters the association between severity of depression and the risk of wound infections. The precise pathophysiology of this correlation is unknown. The correlation between adequate control of CHF and the severity of CHF, and the risk of SSI need to be further investigated. Future research should focus on preoperative assessment of patients with dementia. Ongoing research in HIV/AIDS infection is needed to optimize surgical care of this patient population. 2019 American Academy of Orthopaedic Surgeons

Future Research Factors Associated with an Increased Risk of Infection Future research should evaluate and correlate the severity of renal disease with precise risk of SSI. Additionally, it should use common terminology for renal disease and stratify by dialysis, transplant, and severity of disease. Further studies are needed to identify the relationship between the control of diabetes, Hgb A1C, and the risk of post-operative infection. Research is needed to define the exact correlation between the extent and length of time of tobacco use and the risk of SSI. Determine role of smoking cessation and reducing the risk of SSI. Further study is needed to delineate the duration of smoking cessation and its impact on the occurrence of SSI. Further research is needed to correlate the severity of malnutrition with the concomitant risk of SSI. Also, research into correcting malnutrition and how long after correction will the risk of SSI be reduced. Better definitions of malnutrition should be established via future research. 2019 American Academy of Orthopaedic Surgeons

Future Research Factors Associated with an Increased Risk of Infection Specific analysis between the types, severity and metastasis of cancer needs to be performed to identify the exact correlation between the type of cancer and risk of post op infection. Further research is needed to further delineate the correlation between SSI and hypertension and the preoperative optimization of hypertension and its effect on SSI need to be established. Further research is needed to further delineate the correlation between SSI and liver disease and cirrhosis. 2019 American Academy of Orthopaedic Surgeons

Future Research – Antibiotic Duration As the vast majority of research on antibiotic duration currently centers on the topic of periprosthetic joint infections, future research is needed focusing on other orthopaedic settings, like trauma, pediatrics, and spine. Comparative high-quality studies are needed in order to better delineate the term of antibiotics necessary with implant retention. Also needed are further studies comparing term of antibiotics vs chronic suppression. In addition, future research is needed on antibiotic treatment and duration as related to implant removal. Furthermore, not much data exists on microbes other than Staphylococcus aureus. 2019 American Academy of Orthopaedic Surgeons

Future Research – Rifampin Use Future research is needed to define optimal abx protocols in areas other than joint replacement and organisms other than staph. Very little data exists on the optimal antibiotic regimen in relation to orthopaedic surgical site infection, especially when considering implants outside of joint replacement; in addition, when considering microbes other than Staphylococcus. 2019 American Academy of Orthopaedic Surgeons

This Guideline has been endorsed by the following organizations: 2019 American Academy of Orthopaedic Surgeons

Acknowledgements: Guideline Work Group: Douglas Lundy, Co-Chair Alexander McLaren, MD, Co-Chair Peter F. Sturm, MD Sudheer Reddy, MD Gregory S. Stacy, MD Gwo-Chin Lee, MD Hrayr Basmajian, MD Thomas Fleeter, MD Paul Anderson, MD Sandra B. Nelson, MD Jsoeph Hsu, MD Kim Chillag, DO AAOS Guidelines Oversight Chair: Carter Cassidy, MD AAOS Clinical Practice Guidelines Section Leader: Gregory Brown, MD, PhD AAOS Committee on Evidence-Based Quality and Value Chair: Kevin Shea, MD AAOS Council on Research and Quality Chair: Robert H. Quinn, MD Additional Contributing Members: Douglas Osmon, MD Eric Hume, MD Robert Brophy, MD AAOS Staff: William Shaffer, MD Deborah Cummins, PhD Jayson N. Murray, MA Mary DeMars Mukarram Mohiuddin, MPH Danielle Schulte, MS Peter Shores, MPH Anne Woznica, MLS Kaitlyn Sevarino, MBA 2019 American Academy of Orthopaedic Surgeons
Please Cite Clinical Practice Guideline as: American Academy of Orthopaedic Surgeons Evidence-Based Systematic Literature Review on the Management of Surgical Site Infections. http://www.orthoguidelines.org/topic?id 1022. Published June 9, 2018. 2019 American Academy of Orthopaedic Surgeons

AAOS Systematic Review - Management of Surgical Site Infections Chen, Antonia F. MD, MBA; McLaren, Alex C. MD JAAOS - Journal of the American Academy of Orthopaedic Surgeons: August 15, 2019 - Volume 27 - Issue 16 - p e721–e724 doi: 10.5435/JAAOS-D-18-00643 Case Studies 2019 American Academy of Orthopaedic Surgeons

CASE STUDY - HISTORY A 56-year-old man presented to the emergency department with surgical wound dehiscence and purulence. Three weeks before presentation, he underwent exchange of the tibial polyethylene insert of his right posterior cruciate substituting total knee arthroplasty for mechanical failure of the polyethylene post. His index knee replacement was performed for osteoarthritis 3 years previously, at which time he reported delayed wound healing treated with local wound care. His medical history includes a bicuspid aortic valve, chronic hepatitis C, and abdominal aortic aneurysm treated with transvascular stent, alcohol abuse (30 oz/wk), and cigarette smoking (42 pk years) (recommendation 6 and 8). He is not currently on any prescription or overthe-counter medications, has no known allergies or adverse reactions to medications, and is actively working as a landscape surveyor. 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – PHYSICAL EXAMINATION The patient's height is 5'8'' and weight is 165 pounds (body mass index 25.1 kg/m2), with a temperature of 99 F (recommendation 5), a heart rate of 89 beats per minute, a blood pressure of 131/68, a respiratory rate of 16 breaths per minute, and a blood oxygen saturation of 97% on pulseoximetry. Findings in the right lower extremity include 2 12 cm dehiscence of the central portion of the surgical wound, surrounded by 2 to 6 cm of cutaneous edema, erythema, and desquamating keratin, with areas of purulence and necrosis in the base and along the margins and supra-lateral swelling (recommendation 5) (Figure 1). He had pain with active motion from 0 to 60 of the right knee (recommendation 5) and painless full range of motion of the hip and ankle. The extensor mechanism was intact, and no motor, sensory, perfusion, or pulse deficits were observed. 2019 American Academy of Orthopaedic Surgeons
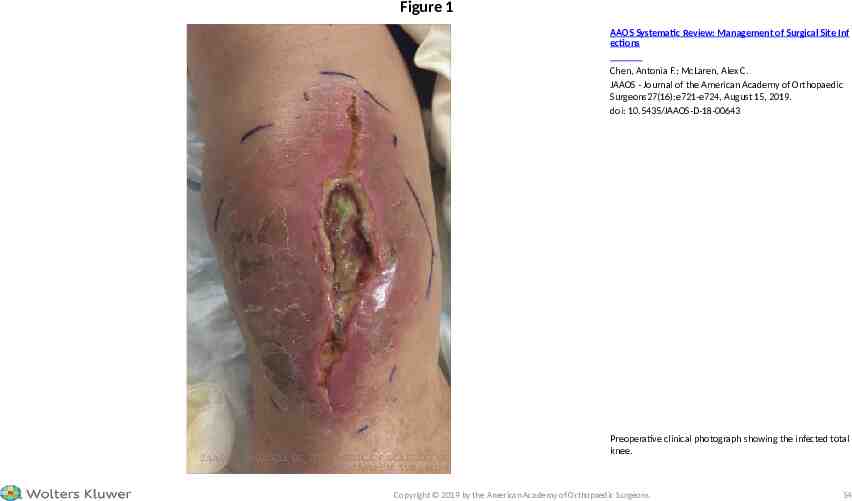
Figure 1 AAOS Systematic Review: Management of Surgical Site Inf ections Chen, Antonia F.; McLaren, Alex C. JAAOS - Journal of the American Academy of Orthopaedic Surgeons27(16):e721-e724, August 15, 2019. doi: 10.5435/JAAOS-D-18-00643 Preoperative clinical photograph showing the infected total knee. Copyright 2019 by the American Academy of Orthopaedic Surgeons. 54
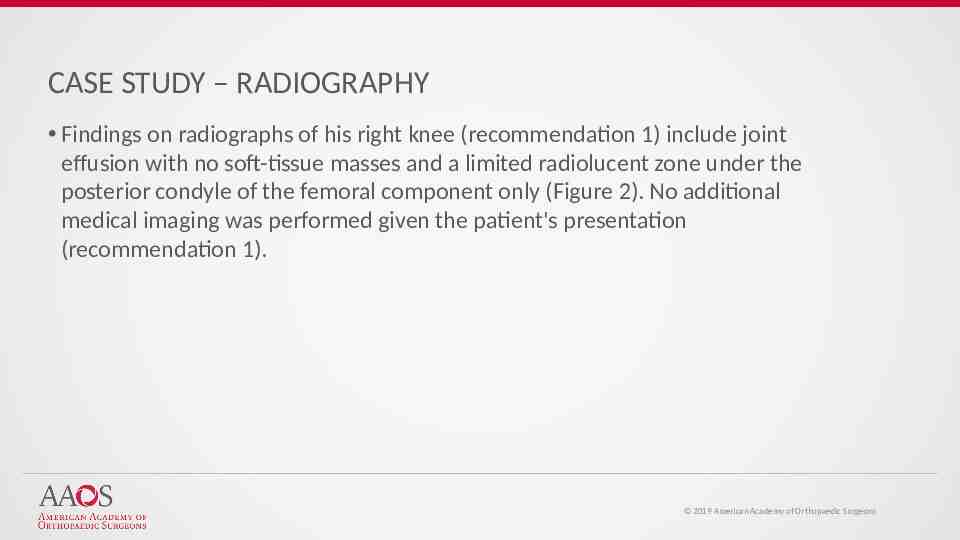
CASE STUDY – RADIOGRAPHY Findings on radiographs of his right knee (recommendation 1) include joint effusion with no soft-tissue masses and a limited radiolucent zone under the posterior condyle of the femoral component only (Figure 2). No additional medical imaging was performed given the patient's presentation (recommendation 1). 2019 American Academy of Orthopaedic Surgeons
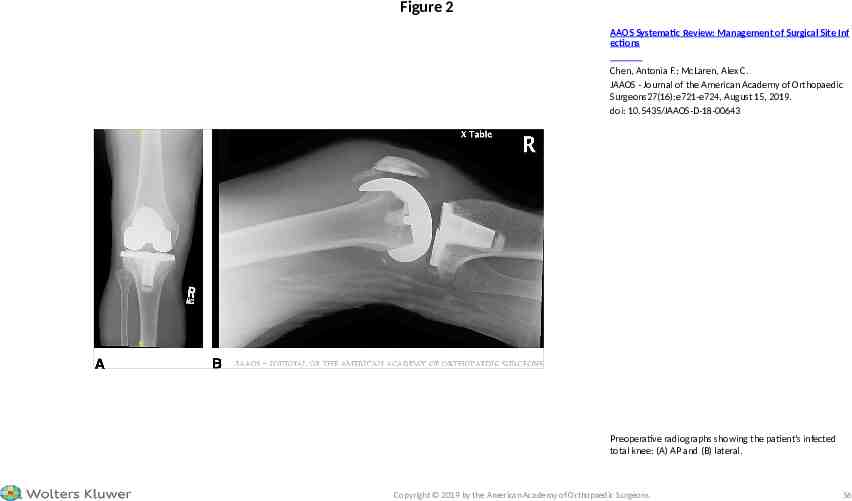
Figure 2 AAOS Systematic Review: Management of Surgical Site Inf ections Chen, Antonia F.; McLaren, Alex C. JAAOS - Journal of the American Academy of Orthopaedic Surgeons27(16):e721-e724, August 15, 2019. doi: 10.5435/JAAOS-D-18-00643 Preoperative radiographs showing the patient's infected total knee: (A) AP and (B) lateral. Copyright 2019 by the American Academy of Orthopaedic Surgeons. 56

CASE STUDY – DIAGNOSIS Group on PJIs, this patient was infected based on four of five positive minor criteria: (1) elevated serum ESR and CRP, (2) elevated synovial fluid WBC, (3) elevated % synovial PMN, and (4) a single positive culture.2 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – SURGICAL MANAGMENT Following informed discussion with the patient, total synovectomy, implant removal with débridement of the underlying bone and surrounding soft tissues, irrigation and placement of a static treatment-dose (tobramycin 3.6 g/vancomycin 2 g/batch) antimicrobial loaded bone cement spacer was performed (Figure 3). In addition to high-dose local antimicrobial delivery, the spacer filled dead space, provided structural stability preventing tissue sheer, achieved bone-spacer interface stability to prevent bone destruction, and maintained the working space/collateral length for the second stage reconstruction. 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – SURGICAL MANAGMENT Intraoperatively, five tissue cultures were taken from the following anatomic sites: two synovium, one posterior capsule, one femoral intramedullary canal, and one tibial canal. No culture swabs were used (recommendation 2). Purulence in the femoral canal was noted. The cultures were incubated aerobically and anaerobically for 14 days (recommendation 2). Because of the risk for atypical/unusual microorganisms, acid-fast bacilli and fungal cultures were performed on select specimens. Acid -fast bacilli and fungal cultures were negative. All cultures were positive for methicillin-sensitive S. aureus and Peptostreptococcus magnus. Medial gastrocnemius flap was performed to cover the 8 16 cm anterior soft-tissue defect on POD 3. 2019 American Academy of Orthopaedic Surgeons
Figure 3 AAOS Systematic Review: Management of Surgical Site Inf ections Chen, Antonia F.; McLaren, Alex C. JAAOS - Journal of the American Academy of Orthopaedic Surgeons27(16):e721-e724, August 15, 2019. doi: 10.5435/JAAOS-D-18-00643 Postoperative radiographs after spacer placement: (A) AP and (B) lateral. Copyright 2019 by the American Academy of Orthopaedic Surgeons. 60
CASE STUDY – POST-DEBRIDEMENT MANAGEMENT Postoperatively, the patient was placed in a knee immobilizer and administered cefazolin 2 g IV every 8 hours for 6 weeks. The gastrocnemius flap healed, and serial serum ESR and CRP levels decreased to 11 mm/hr and 4.4 mg/L, respectively, at 6 weeks post-débridement (recommendation 3 and 4). The patient then underwent a 2-week antibiotic holiday followed by aspiration of the right knee. The posttreatment synovial fluid WBC was 211/mL with 61% neutrophils, and the culture was negative after incubation for 14 days (recommendation 2). The patient underwent reimplantation of his right knee replacement at 10 weeks post-débridement using revision components (Figure 4). Three cultures were taken of soft tissues and the bone adjacent to the spacer during the second stage reimplantation procedure; all were negative at 14 days. 2019 American Academy of Orthopaedic Surgeons
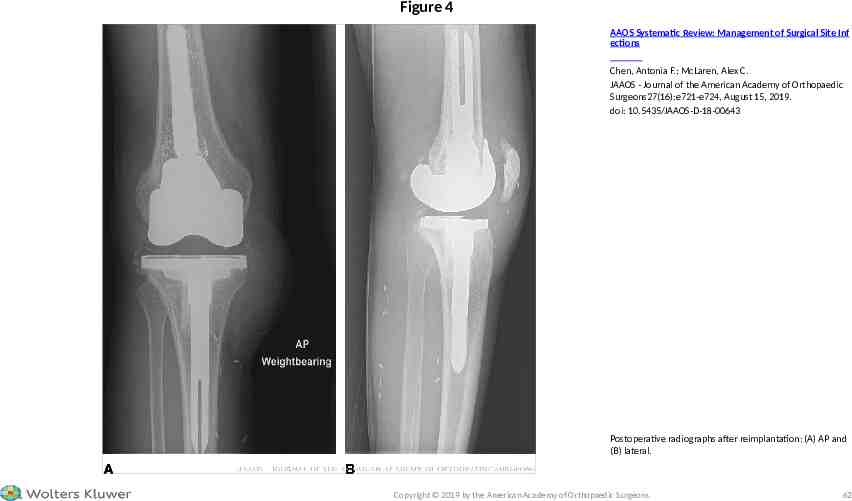
Figure 4 AAOS Systematic Review: Management of Surgical Site Inf ections Chen, Antonia F.; McLaren, Alex C. JAAOS - Journal of the American Academy of Orthopaedic Surgeons27(16):e721-e724, August 15, 2019. doi: 10.5435/JAAOS-D-18-00643 Postoperative radiographs after reimplantation: (A) AP and (B) lateral. Copyright 2019 by the American Academy of Orthopaedic Surgeons. 62

CASE STUDY – POST-DEBRIDEMENT MANAGEMENT After reimplantation, the patient received 14 days of intravenous cefazolin until the intraoperative cultures were reported sterile and was then transitioned to 3 months of oral antimicrobial therapy (not recommendation 9) on the following regimen: (1) oral rifampin 600 milligrams daily for Staphylococcus infection (not Recommendation 10) and (2) trimethoprim/sulfamethoxazol single strength tablets twice a day. He is now off antimicrobials, 1 year after reimplantation, with no signs of infection. 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – DISCUSSION This case highlights several recommendations from the CPG that followed from the Systematic Literature Review on the Management of SSIs. The patient had several independent factors that increased his risk for SSI: alcohol abuse (recommendation 6), cigarette smoking (recommendation 8), and liver disease (hepatitis C) (recommendation 8). Diagnostically, the physical findings were consistent with infection (recommendation 5): pain, soft-tissue appearance. CRP and ESR were both elevated. Recommendation 3 specifically identifies CRP as an independent indicator, whereas ESR needs to be taken in combination with other findings (recommendation 4). During the surgical procedure, tissue biopsies were obtained for culture (recommendation 2) and not swabs, and these cultures were held for a minimum of 14 days (recommendation 2) because of the prolonged incubation times needed to propagate bacteria that have been shed from biofilms. 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – DISCUSSION Post-débridement, the patient received a full 6-week course of parenteral pathogenspecific antimicrobials and the prereconstruction aspiration for culture was delayed for 14 days after the antimicrobials were stopped to maximize the culture yield (recommendation 2). Serum CRP (recommendation 3) and ESR (recommendation 4) were monitored to document a decrease from the pre-débridement levels, before reimplantation. The patient was treated with an extended period of oral antimicrobials (14 weeks) after the second stage reimplantation. This regimen duration is not addressed in recommendation 8, which applies only to patients that have retained implants. 2019 American Academy of Orthopaedic Surgeons

CASE STUDY – REFERENCES 1. American Academy of Orthopaedic Surgeons: Systematic literature review on the management of surgical site infections. 2018. https://www.aaos.org/ssi. 2. Parvizi J, Gehrke T: International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty 2014;29:1331. 2019 American Academy of Orthopaedic Surgeons
Free for both iOS and Android or at www.orthoguidelines.org Provides easy access to all AAOS: Clinical Practice Guidelines Full Guideline PDF’s Appropriate Use Criteria Case Studies Clinician Checklists Impactful Statements Plain Language Summaries Evidence-based Databases Evidence-based Methods, Appraisals and Standards 2019 American Academy of Orthopaedic Surgeons
Easier access to AAOS Guidelines: Sort Alphabetically by Topic Sort Recommendations by Strength (Strong, Moderate, Limited, Consensus) Sort by Stage of Care Search Across all CPGs via a Single Keyword Search Easier Access to Individual Recommendations: View recommendations via shortened titles Access to full recommendation & rationale Links to references (PubMed) 2019 American Academy of Orthopaedic Surgeons
Imaging Search across all CPG and AUC Via a Single Keyword Search 2019 American Academy of Orthopaedic Surgeons
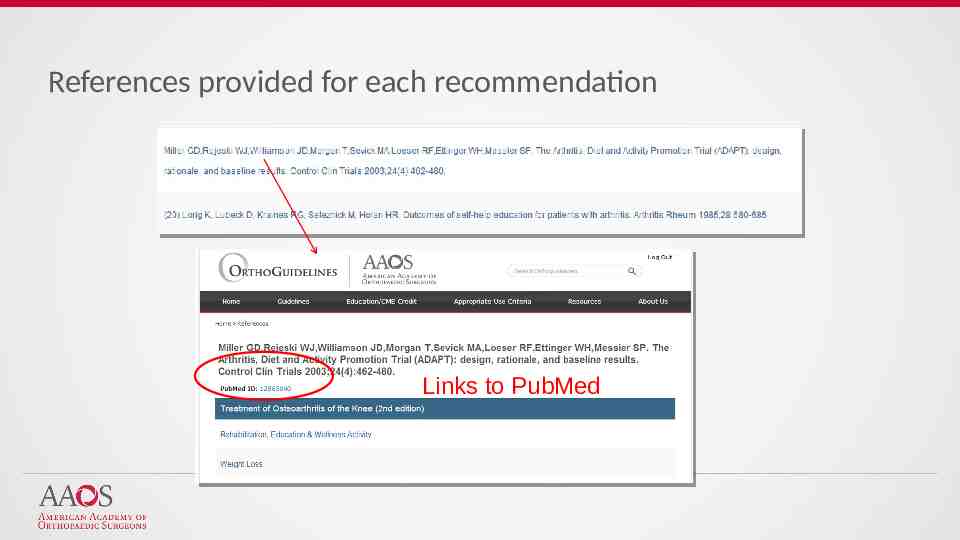
References provided for each recommendation Links to PubMed
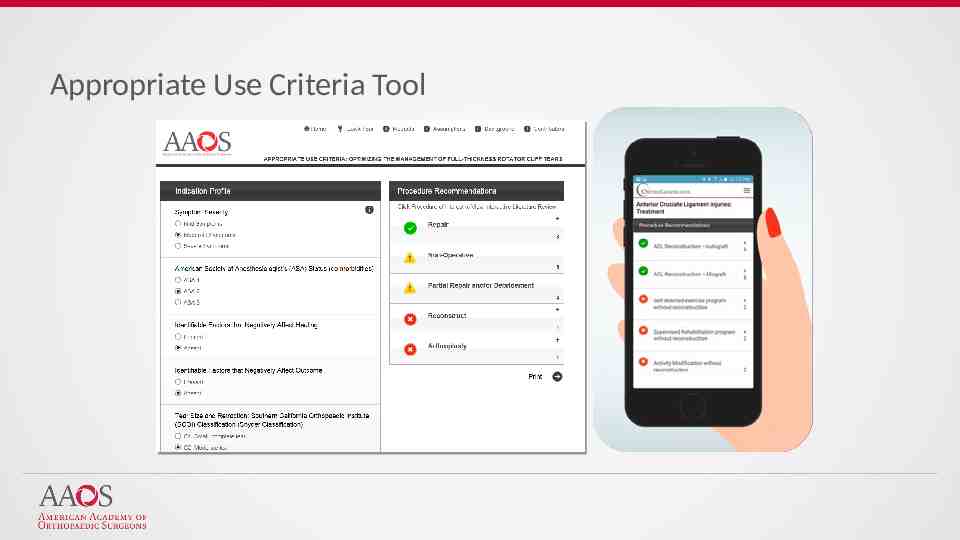
Appropriate Use Criteria Tool

Published Clinical Practice Guidelines Acute Acute Achilles Achilles Tendon Tendon Rupture Rupture Anterior Anterior Cruciate Cruciate Ligament Ligament Injuries Injuries Carpal Carpal Tunnel Tunnel Syndrome Syndrome Distal Distal Radius Radius Fractures Fractures Glenohumeral Glenohumeral Joint Joint Osteoarthritis Osteoarthritis Hip Fractures in the Elderly Hip Fractures in the Elderly Osteoarthritis Osteoarthritis of of the the Hip Hip Osteoarthritis Osteoarthritis of of the the Knee Knee (Arthroplasty) (Arthroplasty) Osteoarthritis Osteoarthritis of of the the Knee Knee (Non-Arthroplasty) (Non-Arthroplasty) Osteochondritis Dissecans Osteochondritis Dissecans Pediatric Pediatric Developmental Developmental Dysplasia Dysplasia of of the the Hip Hip in in infants infants up up to to Six Six Months Months Pediatric Pediatric Diaphyseal Diaphyseal Femur Femur Fractures Fractures Pediatric Pediatric Supracondylar Supracondylar Humerus Humerus Fractures Fractures Periprosthetic Periprosthetic Joint Joint Infections Infections of of the the Hip Hip and and Knee Knee Prevention of Orthopaedic Implant Infections Prevention of Orthopaedic Implant Infections in in Patients Patients Undergoing Undergoing Dental Dental Procedures Procedures Rotator Rotator Cuff Cuff Problems Problems Surgical Surgical Site Site Infections Infections VTE VTE Disease Disease in in Patients Patients Undergoing Undergoing Elective Elective Hip Hip & & Knee Knee Arthroplasty Arthroplasty Tranexamic Acid in Total Joint Arthroplasty (Endorsement) Tranexamic Acid in Total Joint Arthroplasty (Endorsement) Use Use of of Imaging Imaging Prior Prior to to Referral Referral to to aa Musculoskeletal Musculoskeletal Oncologist Oncologist (Endorsement) (Endorsement) For additional information, please visit http://www.orthoguidelines.org/ 2019 American Academy of Orthopaedic Surgeons






