International welcomes submissions OMICSOMICS Journals are welcoming
30 Slides4.28 MB

International welcomes submissions OMICSOMICS Journals are welcoming Submissions that are original and technically so as to serve both the developing world and developed countries in the best possible way. OMICS Journals are poised in excellence by publishing high quality research. OMICS International follows an Editorial Manager System peer review process and boasts of a strong and active editorial board. Editors and reviewers are experts in their field and provide anonymous, unbiased and detailed reviews of all submissions. The journal gives the options of multiple language translations for all the articles and all archived articles are available in HTML, XML, PDF and audio formats. Also, all the published articles are archived in repositories and indexing services like DOAJ, CAS, Google Scholar, Scientific Commons, Index Copernicus, EBSCO, HINARI and GALE. For more details please visit our website: http://omicsonline.org/Submitmanuscript.php

Guidelines to electrochemical methods for low molecular weight biocompounds determination Presented by Aurelia Magdalena Pisoschi Lecturer PhD, University of Agronomic Sciences and Veterinary Medicine of Bucharest, Faculty of Veterinary Medicine, Bucharest, Romania August 2014

Research Interests of the Author: Electrochemical Methods Applied to Food Analysis (Cyclic Voltammetry, Differential Pulse Voltammetry, Amperometry, Potentiometry)

Editorial and Reviewing Activity of the Author Editorial Member of several reputed Journals like Biochemistry Analytical Biochemistry: Current Research and performed significant Reviewing activities for ISI Web of Science indexed Journals like: Talanta, Journal of Electroanalytical Chemistry, Combinatorial Chemistry & High Throughput Screening, Current Pharmaceutical Analysis, Electrochimica Acta, Food Control, Sensors and Actuators B: Chemical, Research on Chemical Intermediates and also for International Data Bases indexed Journals: Oxidants and Antioxidants in Medical Science (Gesdav Foundation, Ankara, Turcia), for several Journals belonging to the Scientific and Academic Publishing Group (American Journal of Biochemistry, American Journal of Chemistry, Food and Public Health, International Journal of Food Science and Nutrition Engineering).

for African Journal of Biotechnology, Agricultural Science Research Journal, International Journal of Biochemistry and Biotechnology, American Journal of Experimental Agriculture, British Journal of Medicine and Medical Research, British Microbiology Research Journal, Education Research Journal, African Journal of Agricultural Research, Basic Research Journal of Medicine and Clinical Sciences, Progress in Color, Colorants and Coatings Journal, International Journal of Biochemistry Research and Review. Author of 40 Papers (among which 3 Reviews, 3 Editorials and a Tutorial) and 4 Books. The Papers have a total number of 133 citations in Google Academic (in conformity to Publish or Perish) and 41 citations in Web of Science indexed Journals.

Lecturer PhD Aurelia Magdalena Pisoschi

Voltammetry Voltammetry is a potentiodynamic technique, based on measuring the current arising from oxidation or reduction reactions at the electrode surface, when a controlled potential variation is imposed. [A.M. Bond, Broadening electrochemical horizons: principles and illustration of voltammetric and related techniques, Oxford University Press, Oxford, New York, 2002] [R.G. Compton and C.E. Banks, Understanding voltammetry (2-nd Edition), World Scientific Publishing Co, New Jersey, London, Singapore, 2010] [A.A. Behfar, N. Sadeghi, B. Jannat, M.R. Oveisi, Determination of L- ascorbic acid in plasma by voltammetric method, Iran. J. Pharm. Res. 2010, Vol. 9, 123]
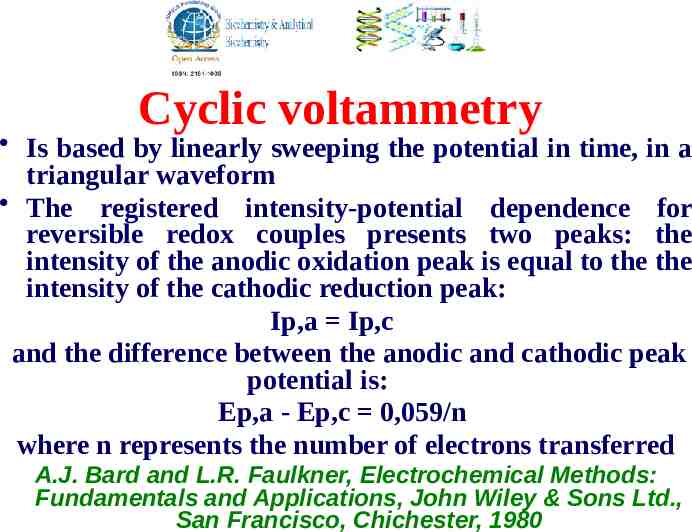
Cyclic voltammetry Is based by linearly sweeping the potential in time, in a triangular waveform The registered intensity-potential dependence for reversible redox couples presents two peaks: the intensity of the anodic oxidation peak is equal to the the intensity of the cathodic reduction peak: Ip,a Ip,c and the difference between the anodic and cathodic peak potential is: Ep,a - Ep,c 0,059/n where n represents the number of electrons transferred A.J. Bard and L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons Ltd., San Francisco, Chichester, 1980

For irreversible redox couples, such as ascorbic acid/dehydroascorbic acid, only the anodic oxidation peak is present. The measured peak current intensity is directly proportional to the concentration value.
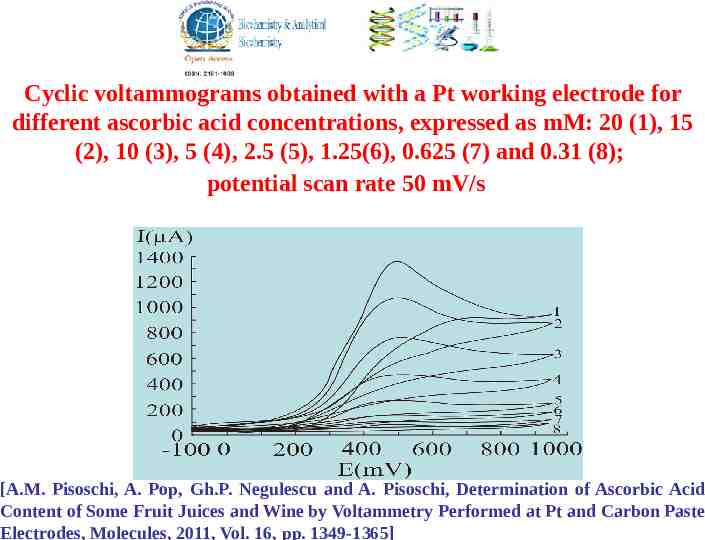
Cyclic voltammograms obtained with a Pt working electrode for different ascorbic acid concentrations, expressed as mM: 20 (1), 15 (2), 10 (3), 5 (4), 2.5 (5), 1.25(6), 0.625 (7) and 0.31 (8); potential scan rate 50 mV/s [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes, Molecules, 2011, Vol. 16, pp. 1349-1365]

The intensity of the anodic peak observes Randles Sevcik dependence, proving that the current is diffusion limited: 5 i p 2,69 10 n 3/ 2 AD 1/ 2 1/ 2 v C n - represents the number of electrons transferred, A - the electrode’s surface, D - the diffusion coefficient, v - the potential scan rate c - the concentration value A.F. Danet, Metode electrochimice de analiza, Editura Stiintifica, Bucuresti, 1996
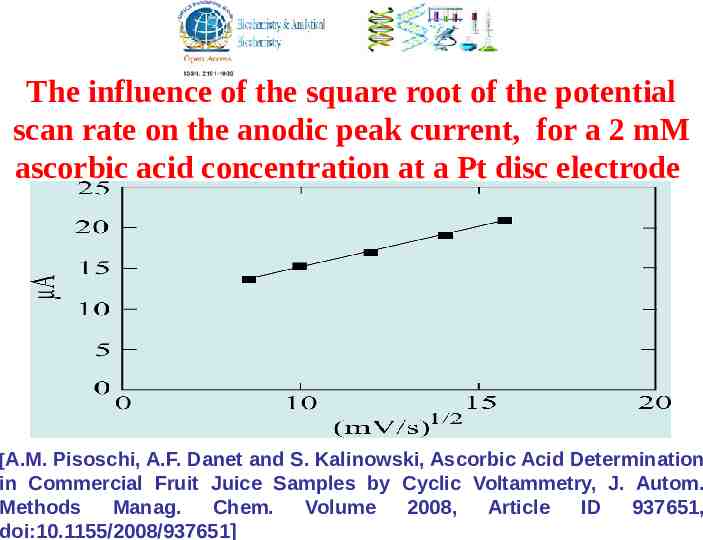
The influence of the square root of the potential scan rate on the anodic peak current, for a 2 mM ascorbic acid concentration at a Pt disc electrode [A.M. Pisoschi, A.F. Danet and S. Kalinowski, Ascorbic Acid Determination in Commercial Fruit Juice Samples by Cyclic Voltammetry, J. Autom. Methods Manag. Chem. Volume 2008, Article ID 937651, doi:10.1155/2008/937651]
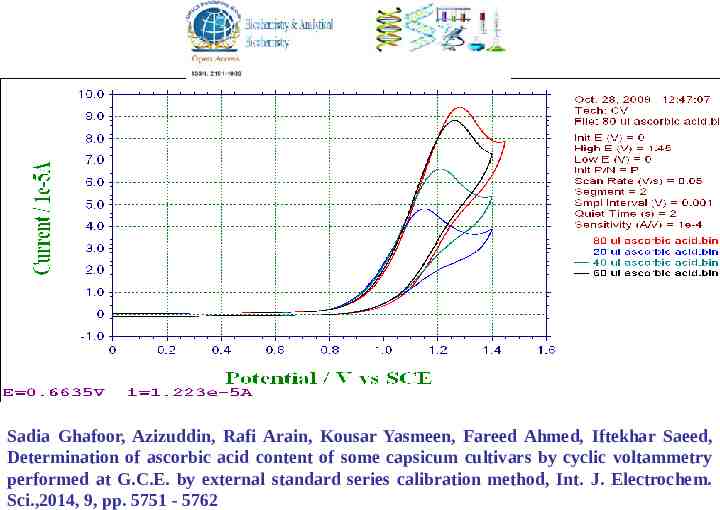
Sadia Ghafoor, Azizuddin, Rafi Arain, Kousar Yasmeen, Fareed Ahmed, Iftekhar Saeed, Determination of ascorbic acid content of some capsicum cultivars by cyclic voltammetry performed at G.C.E. by external standard series calibration method, Int. J. Electrochem. Sci.,2014, 9, pp. 5751 - 5762

Differential pulse techniques In differential pulse techniques, the main advantage consists in actually measuring the Δi/ΔE value, where Δi represents the difference between the values of the current intensities, measured just before pulse application and at the end of the pulse period. [A.F. Danet, Metode electrochimice de analiza, Editura Stiintifica, Bucuresti, 1996] Thus, the charging current which gave some limitations in cyclic techniques with respect to sensitivity, is minimized due to the use of potential step, yielding improved sensitivity, sharper peaks and lower detection limits [A.W. Bott, Practical problems in voltammetry 2. , Curr.Sep., 1993, Vol. 12(1), pp. 1013]
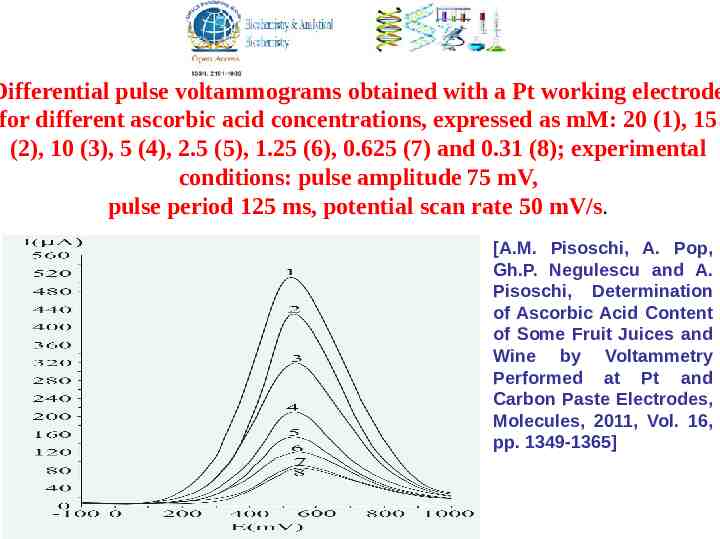
Differential pulse voltammograms obtained with a Pt working electrode for different ascorbic acid concentrations, expressed as mM: 20 (1), 15 (2), 10 (3), 5 (4), 2.5 (5), 1.25 (6), 0.625 (7) and 0.31 (8); experimental conditions: pulse amplitude 75 mV, pulse period 125 ms, potential scan rate 50 mV/s. [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes, Molecules, 2011, Vol. 16, pp. 1349-1365]
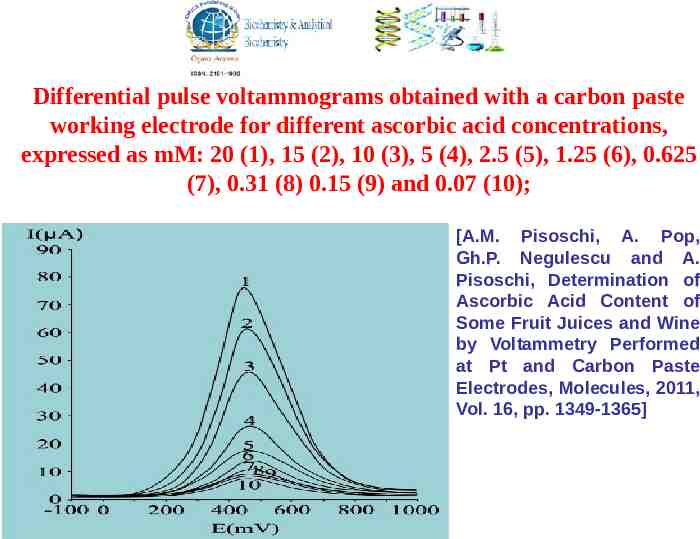
Differential pulse voltammograms obtained with a carbon paste working electrode for different ascorbic acid concentrations, expressed as mM: 20 (1), 15 (2), 10 (3), 5 (4), 2.5 (5), 1.25 (6), 0.625 (7), 0.31 (8) 0.15 (9) and 0.07 (10); [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes, Molecules, 2011, Vol. 16, pp. 1349-1365]
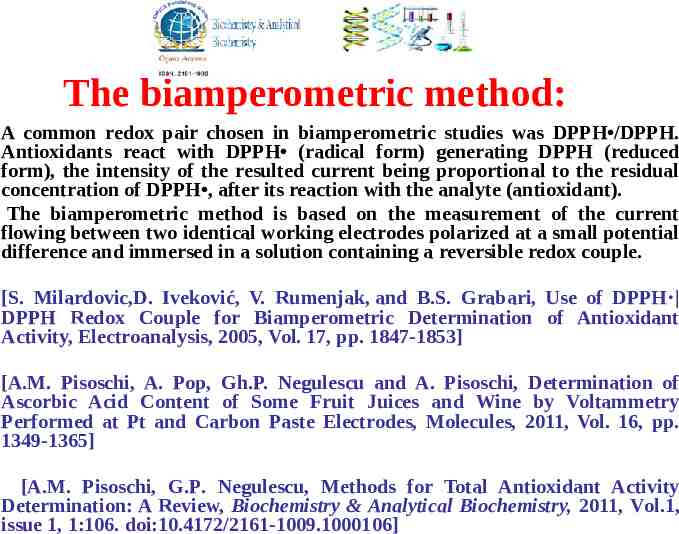
The biamperometric method: A common redox pair chosen in biamperometric studies was DPPH /DPPH. Antioxidants react with DPPH (radical form) generating DPPH (reduced form), the intensity of the resulted current being proportional to the residual concentration of DPPH , after its reaction with the analyte (antioxidant). The biamperometric method is based on the measurement of the current flowing between two identical working electrodes polarized at a small potential difference and immersed in a solution containing a reversible redox couple. [S. Milardovic,D. Iveković, V. Rumenjak, and B.S. Grabari, Use of DPPH DPPH Redox Couple for Biamperometric Determination of Antioxidant Activity, Electroanalysis, 2005, Vol. 17, pp. 1847-1853] [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes, Molecules, 2011, Vol. 16, pp. 1349-1365] [A.M. Pisoschi, G.P. Negulescu, Methods for Total Antioxidant Activity Determination: A Review, Biochemistry & Analytical Biochemistry, 2011, Vol.1, issue 1, 1:106. doi:10.4172/2161-1009.1000106]

The reduction of DPPH at electrode 1 gives rise to a cathodic current, while the oxidation of DPPH at electrode 2 generates an anodic current. Electrode 1: DPPH e- DPPH Electrode 2: DPPH DPPH eThe biamperometric detector response is linear with respect to that constituent of the redox couple which is present in lower concentration. Working conditions were chosen for a DPPH concentration smaller than DPPH concentration, so the cathodic current is limited by the lower concentration of DPPH (radical form) in the indicating mixture. The DPPH /DPPH method was applied to the determination of the total antioxidant capacity in tea, wine and coffee [S. Milardovic, D. Iveković, V. Rumenjak, and B.S. Grabaric, Use of DPPH DPPH Redox Couple for Biamperometric Determination of Antioxidant Activity Electroanalysis, 2005, Vol. 17, pp. 1847-1853], fruit juices [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes Molecules, 2011, Vol. 14, pp. 480-493].
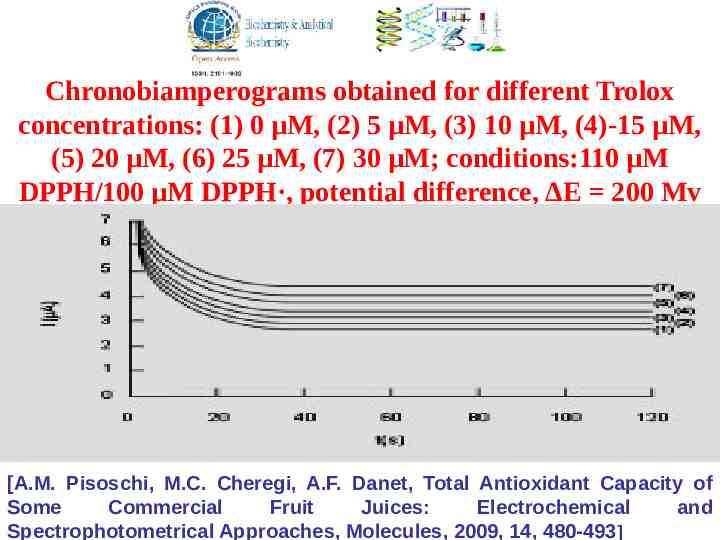
Chronobiamperograms obtained for different Trolox concentrations: (1) 0 μM, (2) 5 μM, (3) 10 μM, (4)-15 μM, (5) 20 μM, (6) 25 μM, (7) 30 μM; conditions:110 μM DPPH/100 μM DPPH·, potential difference, ΔE 200 Mv [A.M. Pisoschi, M.C. Cheregi, A.F. Danet, Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches, Molecules, 2009, 14, 480-493]
Amperometric biosensors The amperometric method implies the application of a constant potential to a working electrode, followed by the measurement of the current intensity. The current flowing through the electrochemical cell is the result of an electron transfer process, either the oxidation or reduction of an electroactive analyte. Amperometric biosensors rely on the reduction or oxidation of an electroactive species, which is either product, or co-substrate of an enzyme reaction. Therefore, the role of the biocatalyst is to generate/consume an electroactive species, which can be stoichiometrically correlated to the analyte concentration. The biorecognition elements are represented by enzymes, nucleic acids, lectins or whole cells. [Blum L, Coulet P (Edts.) (1991) Biosensor principles and application, Marcel Dekker, Inc, New York] [Scheller F, Schubert F (1992) Biosensors, Elsevier, Amsterdam [Dzyadevych SV, Arkhypova VN, Soldatkin, AP, Elskaya AV, Martelet C, et al. (2008) Amperometric enzyme biosensors: Past, present and future, IRBM Vol. 29, pp. 171-180] [Pisoschi A.M., Biosensors as Bio-Based Materials in Chemical Analysis: A Review., J. Biobased Mater. Bio., 2013, Vol. 7, pp. 19-38]

Potentiometric biosensors In potentiometric biosensors, the biochemical reaction involves an ion concentration variation, which is consecutively sensed by the transducer. The most commonly used transducer in the construction of potentiometric enzyme sensors was the glass-pH electrode, as many enzyme reactions involving key analytes (glucose, urea, triglycerides, penicillin, pesticides) take place with either proton generation or consumption. [Blum L, Coulet P (Edts.) (1991) Biosensor principles and application, Marcel Dekker, Inc, New York] [Scheller F, Schubert F (1992) Biosensors, Elsevier, Amsterdam] [Pisoschi A.M., Biosensors as Bio-Based Materials in Chemical Analysis: A Review., J. Biobased Mater. Bio., 2013, Vol. 7, pp. 19-38] [Pisoschi A.M., Determination of Several Food Additives and Ingredients by Electrochemical Techniques, Biochemistry & Analytical Biochemistry: Current Research, 2013, vol. 2 issue 3, e140 doi: 10.4172/2161-1009.1000e140]

Apparatus Schematic representation of the experimental setup [A.M. Pisoschi, A. Pop, Gh.P. Negulescu and A. Pisoschi, Determination of Ascorbic Acid Content of Some Fruit Juices and Wine by Voltammetry Performed at Pt and Carbon Paste Electrodes, Molecules, 2011, Vol. 16, pp. 13491365] KSP potentiostatgalvanostat, made by prof. Slawomir Kalinowski, Warmia and Mazury University, Olsztyn, Poland http://debiany.pl/ksp/ index.html

Types of electrodes working electrodes: Pt, Au, glassy carbon, carbon paste, mercury etc.; represent the electrodes at which the reaction of interest takes place. reference electrodes: Ag/AgCl, calomel, mercury/ mercurous sulphate (Hg/HgSO4); they settle the potential value against which, other potentials may be measured. auxiliary electrodes: generally fabricated from electrochemically inert materials: gold, platinum, or carbon; together with the working electrode, an auxiliary electrode provides the circuit over which the current can be applied or measured. A.J. Bard and L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons Ltd., San Francisco, Chichester, 1980

Enzyme electrodes In potentiometric biosensors, the biocatalytical element is immobilized in semipermeable membranes such as cellophane, nitrocellulose or nylon, subsequently fixed on the surface of the transducer, the most often encountered being the glass-pH electrode, but also, ion selective field effect transistors, metal, metal oxide transducers or nanoparticles-based. In amperometric biosensors, the enzyme membrane can be fixed on the transducer’s surface, the first used being the oxygen electrode. Pt transducers for hydrogen peroxide can be also employed, and in this case the diminution in the working potential can be obtained by the use of redox mediators. Also, amperometric enzyme sensors based on polymeric films, carbon nanotubes, various composites or by using the screen-printing technique were developed, leading to improved analytical performances. [Pisoschi A.M., Determination of Several Food Additives and Ingredients by Electrochemical Techniques, Biochemistry & Analytical Biochemistry: Current Research, 2013, vol. 2 issue 3, e140 doi: 10.4172/2161-1009.1000e140] [Pisoschi A.M., 4. A.M. Pisoschi, Glucose determination by biosensors editorial, Biochemistry & Analytical Biochemistry: Current Research, 2012, vol.1. issue 6, 1:e119. doi:10.4172/2161-1009.1000e119 ]

Supporting electrolyte – general aspects role: to minimize solution resistance absence: reduce the accuracy of measurements concentration: support ratio (conc. electrolyte/conc. analyte) 30 exemples: solutions of - acids (citric, HCl, H2SO4 etc.); - bases ( NaOH, KOH etc.); - sels (chlorides, nitrates, etc.) - buffers (phosphate, tartrate etc.); - nonaqueous solutions (acetonitrile, DMSO, DMF etc.).
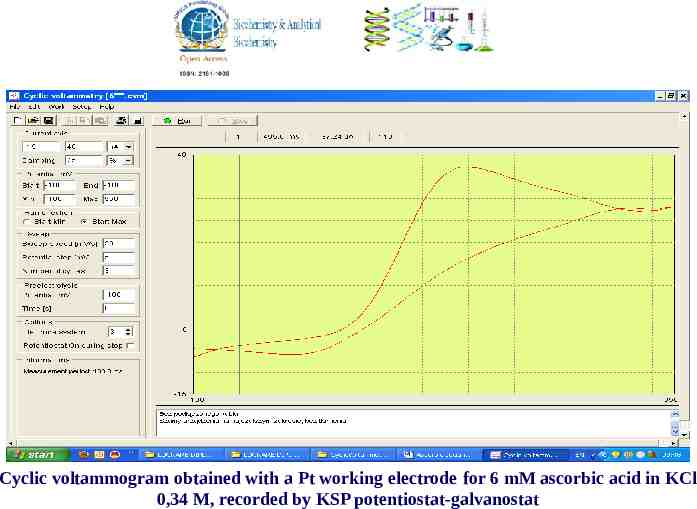
Cyclic voltammogram obtained with a Pt working electrode for 6 mM ascorbic acid in KCl 0,34 M, recorded by KSP potentiostat-galvanostat

Conclusions The voltammetric methods (cyclic or differential pulse voltammetry) for ascorbic acid and other low molecular weight antioxidants are characterized by sensitivity, rapidity and reproducibility. Cyclic voltammetry, although applied with reliable results in various media (foodstuffs, pharmaceuticals, biological fluids), can suffer from restricted values detection limit (105 M), while step techniques like differential pulse and square wave voltammetry, result in lower detection limit (below 10-7 M) and better resolution, due to minimizing charging current. Amperometric and potentiometric biosensors benefit from the specificity imparted by the biorecognition element.
Biochemistry and Analytical Biochemistry Related Journals Journal of Plant Biochemistry & Physiology Arrow Bio molecular Research & Therapeutics Arrow Biochemistry & Pharmacology: Open Access Arrow Biochemistry & Physiology: Open Access

OMICS International Open Access Membership OMICS publishing Group Open Access Membership enables academic and research institutions, funders and corporations to actively encourage open access in scholarly communication and the dissemination of research published by their authors. For more details and benefits, click on the link below: http://omicsonline.org/membership.php

For more Upcoming Conferences visit: http://www.conferenceseries.com/






