Heating & Cooling Curves Unit 7: Lesson #3
22 Slides1.50 MB

Heating & Cooling Curves Unit 7: Lesson #3

Heating/Cooling Curves: Show the change of state over time that occurs with the addition or removal of heat energy

What happens when you add heat to a solid? HEATING curve for water Temp Heat can be transferred even if there is no change in state 100 0 Ice at –30.0 C absorbs heat but stays SOLID while temperature rises to 0 C. Time

What happens as a solid melts? HEATING curve for water Temp 100 0 At 0 C ice begins to melt Time During a phase change, the temperature remains CONSTANT. This is because the heat being added is used to break IMFs holding the molecules together not to raise the temperature.
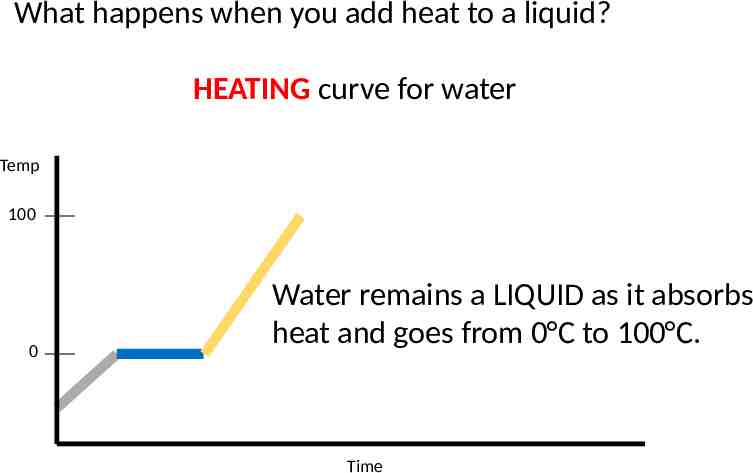
What happens when you add heat to a liquid? HEATING curve for water Temp 100 0 Water remains a LIQUID as it absorbs heat and goes from 0 C to 100 C. Time
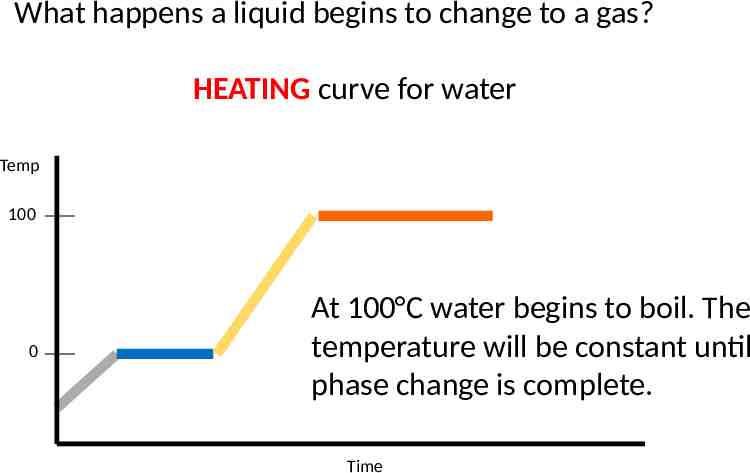
What happens a liquid begins to change to a gas? HEATING curve for water Temp 100 0 At 100 C water begins to boil. The temperature will be constant until phase change is complete. Time
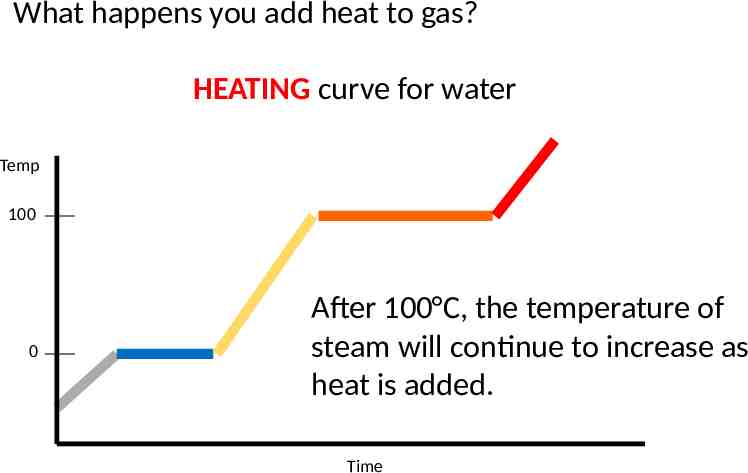
What happens you add heat to gas? HEATING curve for water Temp 100 0 After 100 C, the temperature of steam will continue to increase as heat is added. Time
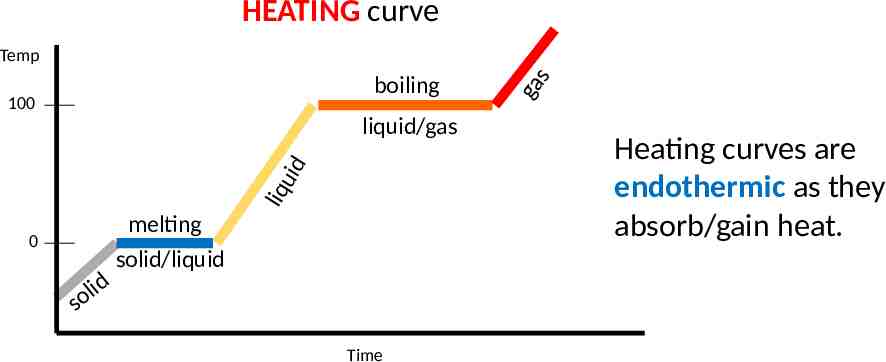
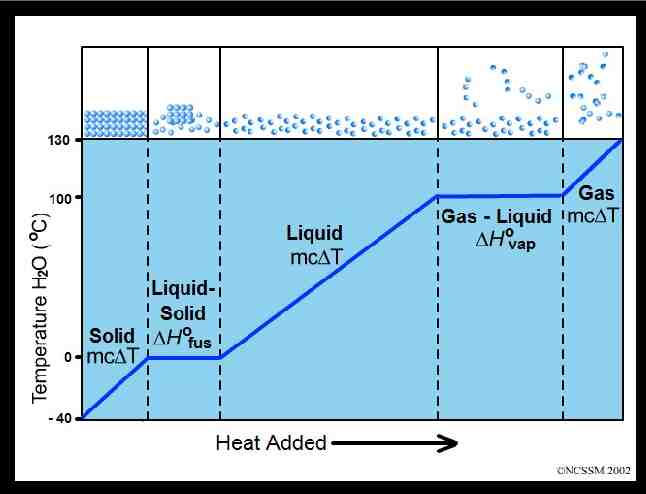
HEATING curve boiling 100 liq uid liquid/gas 0 d i l so melting solid/liquid Time ga s Temp Heating curves are endothermic as they absorb/gain heat.
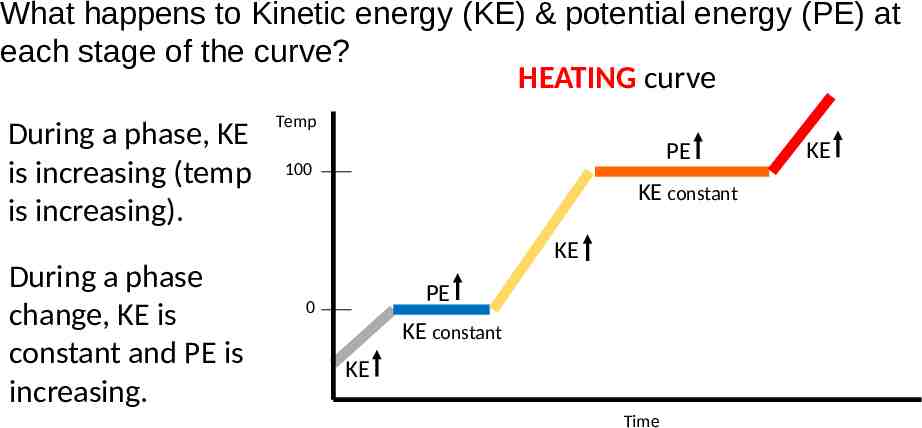
What happens to Kinetic energy (KE) & potential energy (PE) at each stage of the curve? HEATING curve During a phase, KE is increasing (temp is increasing). During a phase change, KE is constant and PE is increasing. Temp PE 100 KE constant KE PE 0 KE constant KE Time KE
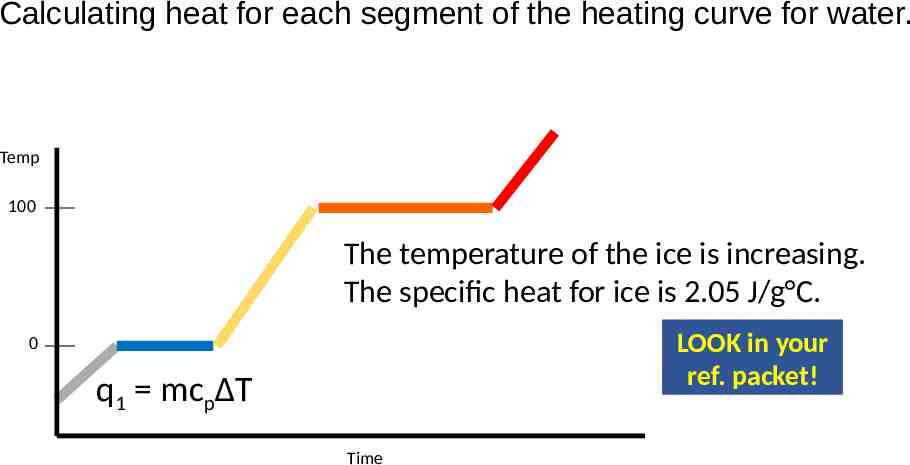
Calculating heat for each segment of the heating curve for water. Temp 100 The temperature of the ice is increasing. The specific heat for ice is 2.05 J/g C. LOOK in your ref. packet! 0 q1 mcpΔT Time
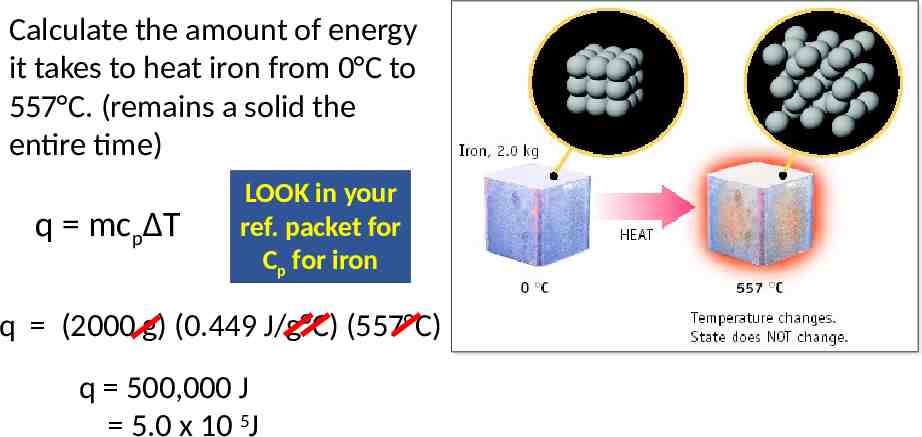
Calculate the amount of energy it takes to heat iron from 0 C to 557 C. (remains a solid the entire time) q mcpΔT LOOK in your ref. packet for Cp for iron q (2000 g) (0.449 J/g C) (557 C) q 500,000 J 5.0 x 10 5J
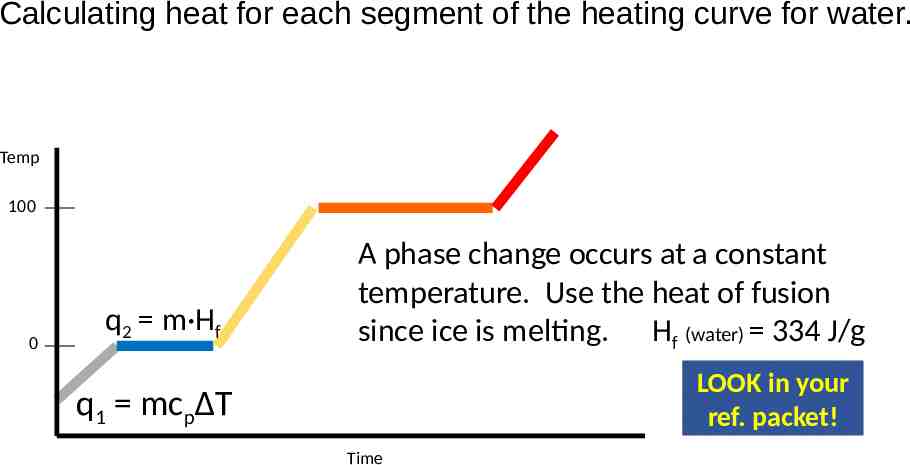
Calculating heat for each segment of the heating curve for water. Temp 100 0 q2 m·Hf A phase change occurs at a constant temperature. Use the heat of fusion since ice is melting. Hf (water) 334 J/g LOOK in your ref. packet! q1 mcpΔT Time
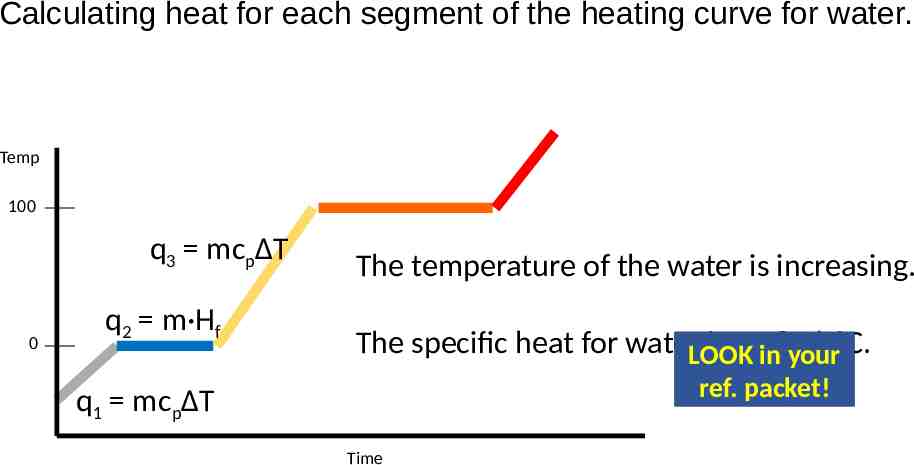
Calculating heat for each segment of the heating curve for water. Temp 100 q3 mcpΔT 0 q2 m·Hf The temperature of the water is increasing. The specific heat for waterLOOK is 4.18 J/g C. in your ref. packet! q1 mcpΔT Time
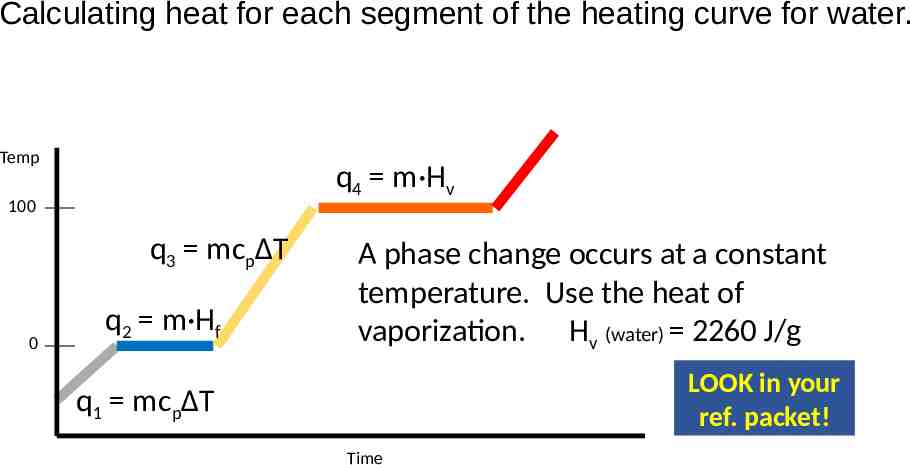
Calculating heat for each segment of the heating curve for water. Temp q4 m·Hv 100 q3 mcpΔT 0 q2 m·Hf A phase change occurs at a constant temperature. Use the heat of vaporization. Hv (water) 2260 J/g LOOK in your ref. packet! q1 mcpΔT Time
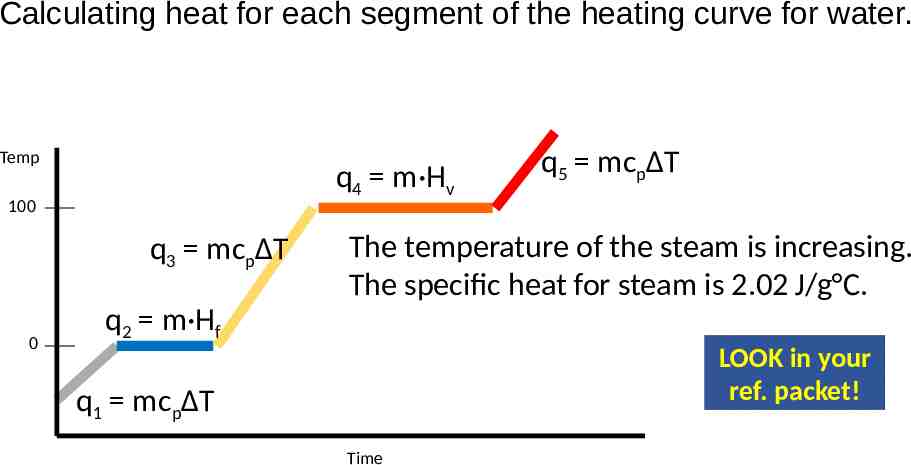
Calculating heat for each segment of the heating curve for water. Temp q4 m·Hv 100 q3 mcpΔT 0 q5 mcpΔT The temperature of the steam is increasing. The specific heat for steam is 2.02 J/g C. q2 m·Hf LOOK in your ref. packet! q1 mcpΔT Time
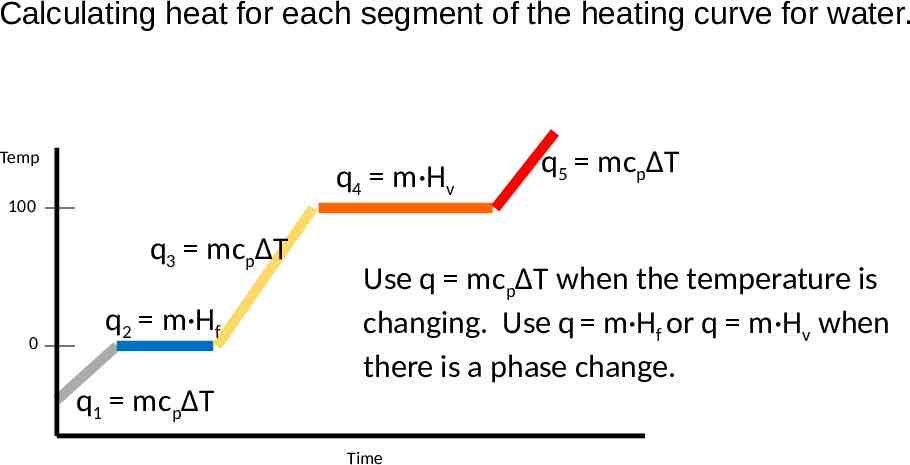
Calculating heat for each segment of the heating curve for water. Temp q4 m·Hv 100 q3 mcpΔT 0 q2 m·Hf q5 mcpΔT Use q mcpΔT when the temperature is changing. Use q m·Hf or q m·Hv when there is a phase change. q1 mcpΔT Time
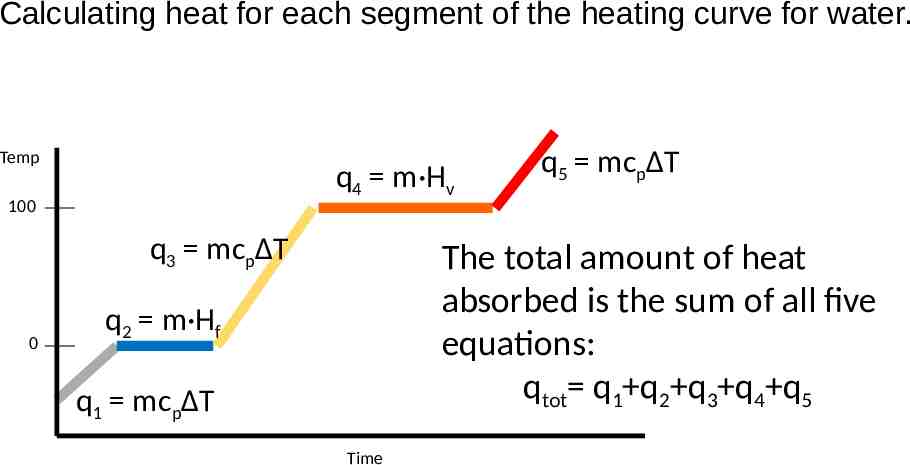
Calculating heat for each segment of the heating curve for water. Temp q4 m·Hv 100 q3 mcpΔT 0 q5 mcpΔT The total amount of heat absorbed is the sum of all five equations: qtot q1 q2 q3 q4 q5 q2 m·Hf q1 mcpΔT Time
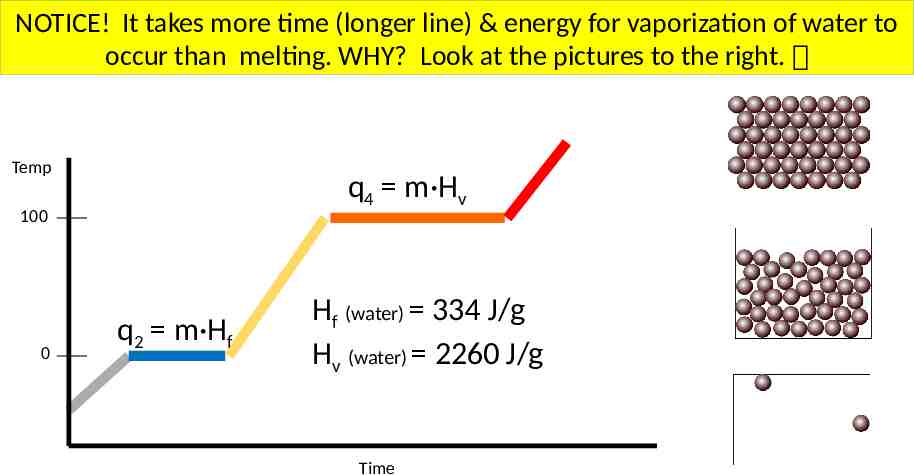
NOTICE! It takes more time (longer line) & energy for vaporization of water to occur than melting. WHY? Look at the pictures to the right. Temp q4 m·Hv 100 0 q2 m·Hf Hf (water) 334 J/g Hv (water) 2260 J/g Time
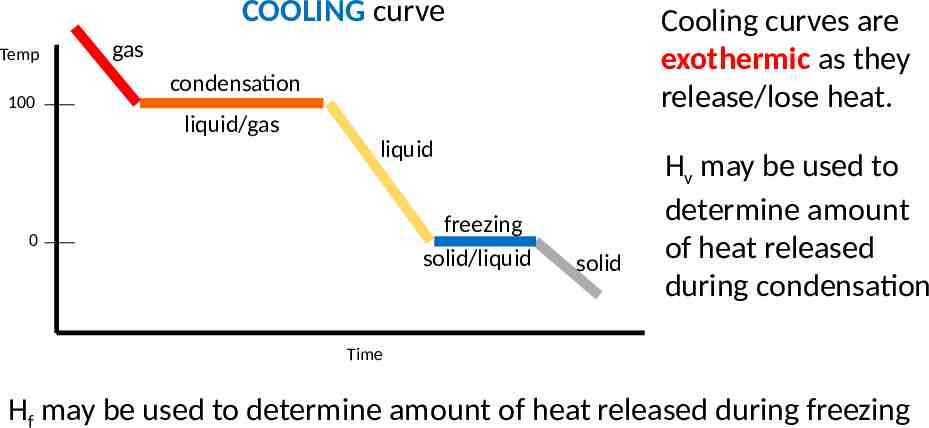
COOLING curve Temp 100 Cooling curves are exothermic as they release/lose heat. gas condensation liquid/gas liquid freezing solid/liquid 0 solid Hv may be used to determine amount of heat released during condensation Time Hf may be used to determine amount of heat released during freezing
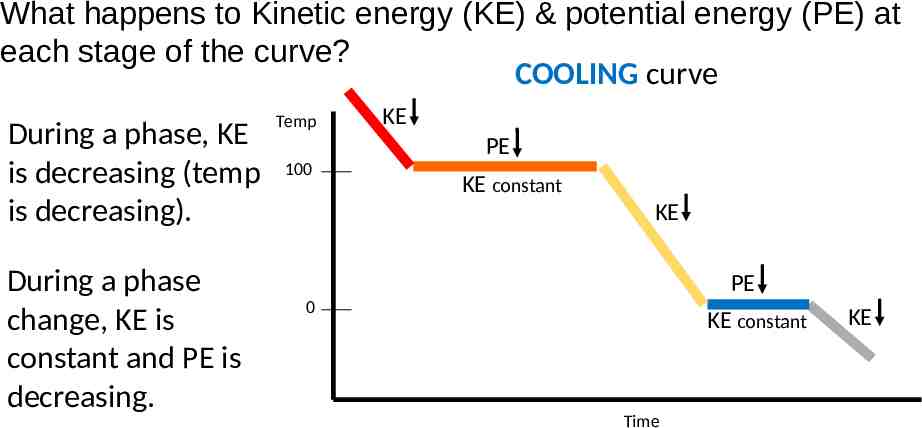
What happens to Kinetic energy (KE) & potential energy (PE) at each stage of the curve? COOLING curve During a phase, KE is decreasing (temp is decreasing). During a phase change, KE is constant and PE is decreasing. Temp KE PE 100 KE constant KE PE 0 KE constant Time KE
How much heat is released when 275 grams of water freezes? q m·Hf q (275 g)(334 J/g) q 91,850 J q 91.9 kJ







