Energy Conservation(cont.) Example: Superheated water vapor
6 Slides318.00 KB
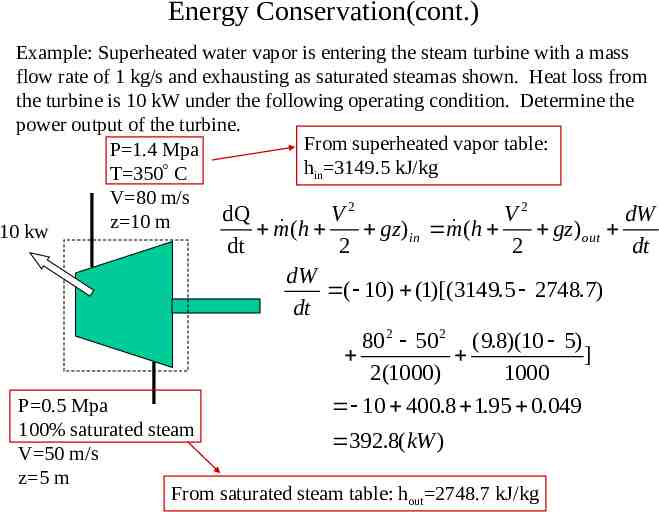
Energy Conservation(cont.) Example: Superheated water vapor is entering the steam turbine with a mass flow rate of 1 kg/s and exhausting as saturated steamas shown. Heat loss from the turbine is 10 kW under the following operating condition. Determine the power output of the turbine. From superheated vapor table: P 1.4 Mpa hin 3149.5 kJ/kg T 350 C V 80 m/s 2 2 dQ V V dW z 10 m m ( h gz ) m ( h gz ) 10 kw in out dt 2 2 dW ( 10) (1)[(3149.5 2748.7) dt 80 2 50 2 (9.8)(10 5) ] 2(1000) 1000 10 400.8 1.95 0.049 P 0.5 Mpa 100% saturated steam 392.8( kW ) V 50 m/s z 5 m From saturated steam table: hout 2748.7 kJ/kg dt

Saturated Steam f-liquid phase g-vapor phase These properties are all dependent: specify one to determine all (because they are in a saturation state) Liquid and vapor phases coexist, the total mass of the mixture, m, is the sum of the liquid mass and the vapor mass: m mf mg, The ratio of the mass of vapor to the total mass is called the quality of the mixture: x mg/m

Total volume is the sum of liquid volume and vapor volume: V Vf Vg mfvf mgvg, where v is the specific volume or 1/ V m(1/ ) mv] V/m v Vf/m Vg/m (mf/m)vf (mg/m)vg [(m-mg)/m]vf (mg/m)vg (1-x)vf xvg vf x(vg-vf) vf xvfg, where vfg vg-vf Similarly, all other saturated thermodynamic properties can be expressed in the same manner: Ex: internal energy: u (1-x)uf xug uf x(ug-uf) uf xufg

p (Mpa) Saturated Steam Table hfg (kJ/kg) hf (kJ/kg) hg (kJ/Kg) hg(p 0.5 Mpa) 2746.4 (2758.1-2746.4)/(0.6178-0.4758)*(0.5-0.4758) 2748.4 kJ/kg for 100% quality saturated vapor Example: If the quality is 50% and the temperature is 150 C hf 632.2, hfg 2114.2, hg 2746.4 h (1-x) hf x hg (1-0.5)(632.2) 0.5(2746.4) 1689.3 (kJ/kg)

Superheated Steam h(p 1MPa, T 350 C) 3157.7 kJ/kg h(p 1.5MPa, T 350 C) 3147.4 kJ/kg h(p 1.4MPa, T 350 C) 3157.7 (3147.7-3157.7)*(0.4/0.5) 3149.7 (kJ/kg)

Compressed Liquid Similar to the format of the superheated vapor table In general, properties are not sensitive to pressure, therefore, can treat the compressed liquid as saturated liquid at the given TEMPERATURE. Given: P and T: v v f @ T , u u f @ T , s s f @ T But not h, since h u pv, and it depends more strongly on p. It can be approximated as h h v ( p p ) f @T f sat