Clinical Trials
12 Slides688.50 KB

Clinical Trials

Clinical Trial New Drug Development Timeline Source: FDA/Center for Drug Evaluation and Research www.fda.gov/Drugs/DevelopmentApprovalProcess/SmallBusinessAssistance/ucm053131.htm

Clinical Trials Research and Development R&D involves initial synthesis and analysis of a promising pharmaceutical OR development and analysis of a biopharmaceutical produced in living cells. On upcoming slides, the word “drug” applies both to pharmaceuticals and to biopharmaceuticals.

Clinical Trials Investigational New Drug Application The application to the FDA to request permission to begin human testing is called an Investigational New Drug application or IND. The IND permits the use of an investigational new drug for the sole purpose of conducting clinical trials.

Clinical Trials Phase 1 Drug is tested for its interaction with the human system. How is the drug absorbed How is the drug distributed in the body How is the drug metabolized by the body Trials usually involve normal, healthy volunteers and take about a year to complete.

Clinical Trials Phase 2 Pilot student to begin to define the effectiveness and safety of the drug in patients with the disease or condition to be treated, diagnosed or prevented. Testing the various doses of the drug and dosing regimens

Clinical Trials Phase 3 Expanded clinical trials Designed to Gather additional evidence of effectiveness for specific interactions Better understand safety and drug-related adverse effects

Clinical Trials Phase 4 Studies that occur after a drug has received approval from the U.S. Food and Drug Administration to be marketed Performed to determine the incidence of adverse reactions Determine the long-term effect of the drug To study a patient population not previously studied For marketing comparisons against other products and users
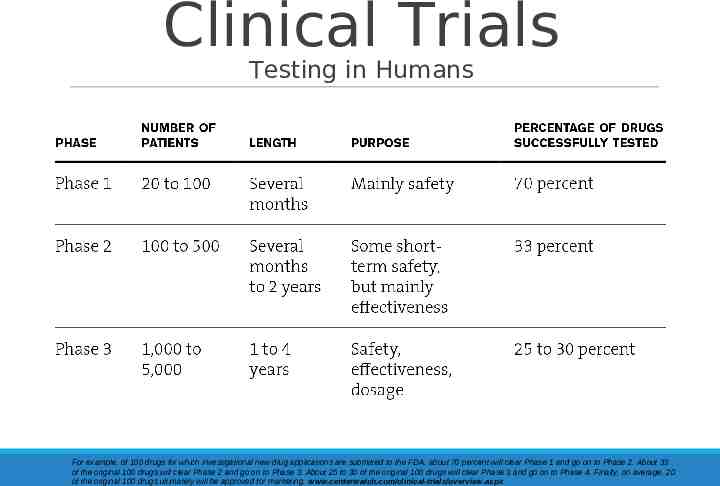
Clinical Trials Testing in Humans For example, of 100 drugs for which investigational new drug applications are submitted to the FDA, about 70 percent will clear Phase 1 and go on to Phase 2. About 33 of the original 100 drugs will clear Phase 2 and go on to Phase 3. About 25 to 30 of the original 100 drugs will clear Phase 3 and go on to Phase 4. Finally, on average, 20 of the original 100 drugs ultimately will be approved for marketing. www.centerwatch.com/clinical-trials/overview.aspx

Clinical Trials New Drug Application (NDA) Submitted to the FDA if Phase 1, 2 and 3 trials indicate the drug is safe and effective Comprehensive statement with information about the drug’s chemical structure, scientific rationale and purpose of the drug therapy, preclinical and other laboratory results, all human clinical testing data, drug formulation and production details, and proposed labeling 1000s of pages which are submitted electronically.

Clinical Trials New Drug Application (NDA) FDA has 60 to conduct a preliminary review and decide if it has enough information to proceed with the NDA review FDA required to make a decision within 180 days of the date the NDA is submitted FDA and company can create a mutual agreement to lengthen the time frame NDA decision-making process can take anywhere from 2 months to 7 years — the average time is 2 years May require minor or major changes of the NDA or additional clinical studies and may inspect the production, testing and packaging facilities to ensure they are compliant with regulations

Clinical Trials Post-marketing surveillance Monitor the ongoing safety of marketed drugs by reassessing drug risk based on New data collected after the drug is marketed By recommending ways of trying to most appropriately manage that risk Includes adverse reaction reporting by the medical community of the pharmaceutical company that markets the drugs Periodic sampling and testing of the drug Periodic inspections of the manufacturing and distribution process