Bonding Types of Bonds Polarity Ionic radius and ionic character
25 Slides836.00 KB
Bonding Types of Bonds Polarity Ionic radius and ionic character
Why form bonds? 1. To get to a noble gas configuration (octet rule) – Ionic bonds (metal nonmetal) – Metals want to lose their valence electrons Nonmetals want to gain electrons Covalent bonds (nonmetals) Both nonmetals want to share to reach a noble gas configuration 2. To get to the lowest possible energy

Exceptions to the Octet Rule Hydrogen and helium want only 2 Beryllium (metalloid) wants only 4 Boron wants only 6 3rd energy level or below could be expanded if necessary – bonds using electrons in d orbitals
Reminders Electronegativity – How tightly an atom holds onto electrons in a bond – A measure of the attraction of an atom to another atom’s electrons in a bond
Reminders Ionic bond – Transfer of electrons Covalent bond – Sharing of electrons (a covalent bond is always TWO shared electrons) Polarity – Unequal distribution of charge
Types of Bonds Based on the DIFFERENCE in electronegativity – System by Linus Pauling (1932) – The more electronegative atom pulls the electrons in a bond
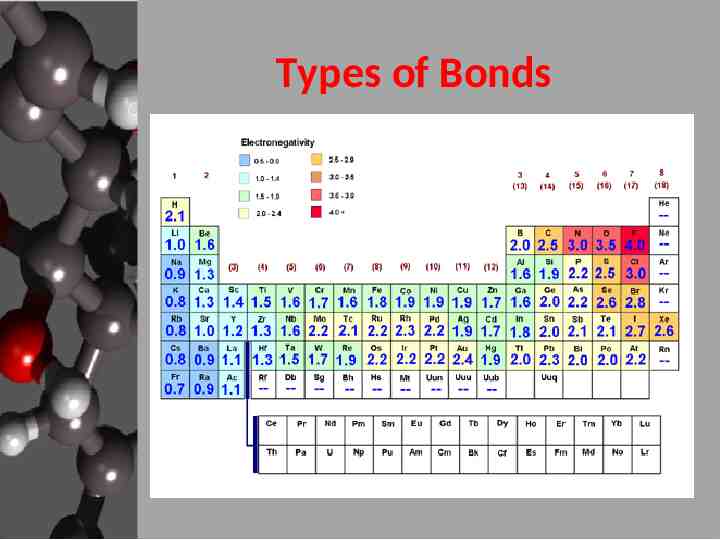
Types of Bonds
Types of Bonds Pauling’s system – Set fluorine as 4.0 – Differences in electronegativity 0.4 nonpolar covalent 0.4 – 2.0 polar covalent 2.0 ionic
Types of Bonds What it really means – Same atom bonded to itself NONPOLAR – Two different nonmetals bonded together POLAR The larger the difference, the more polar the bond – Metal and nonmetal IONIC
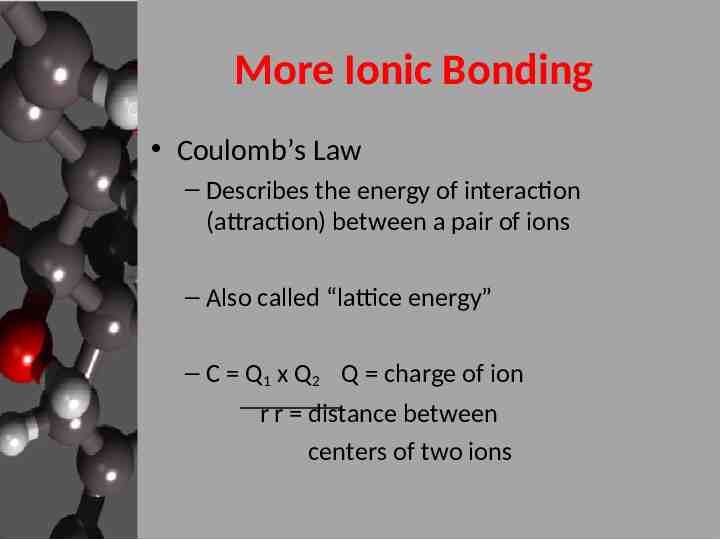
More Ionic Bonding Coulomb’s Law – Describes the energy of interaction (attraction) between a pair of ions – Also called “lattice energy” – C Q1 x Q2 Q charge of ion r r distance between centers of two ions
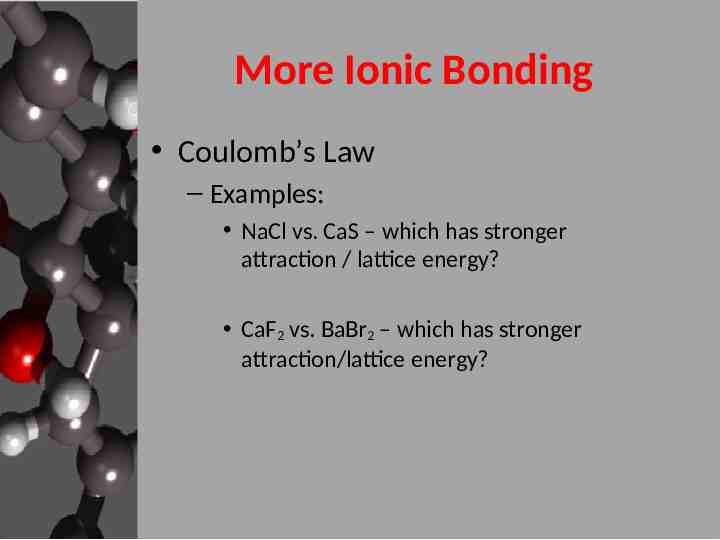
More Ionic Bonding Coulomb’s Law – Examples: NaCl vs. CaS – which has stronger attraction / lattice energy? CaF2 vs. BaBr2 – which has stronger attraction/lattice energy?
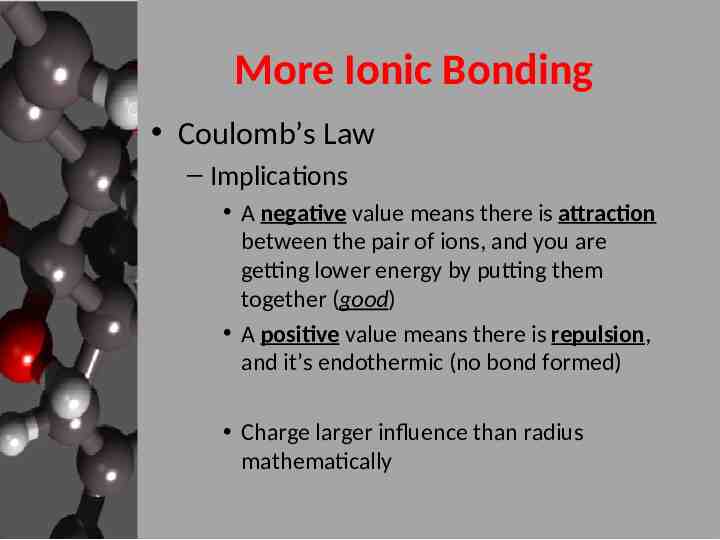
More Ionic Bonding Coulomb’s Law – Implications A negative value means there is attraction between the pair of ions, and you are getting lower energy by putting them together (good) A positive value means there is repulsion, and it’s endothermic (no bond formed) Charge larger influence than radius mathematically

More Ionic Bonding Ionic character – Measure of how ionic something is – Can use locations on periodic table to predict Shown – Which one is most ionic? NaF LiBr KBr NaI
More Covalent Bonding Two nonmetals sharing electrons Two different things bonded together is a polar bond Overall molecule may be polar or nonpolar based on symmetry

Bond Length Diagrams http://phet.colorado.edu/en/simulati on/atomic-interactions
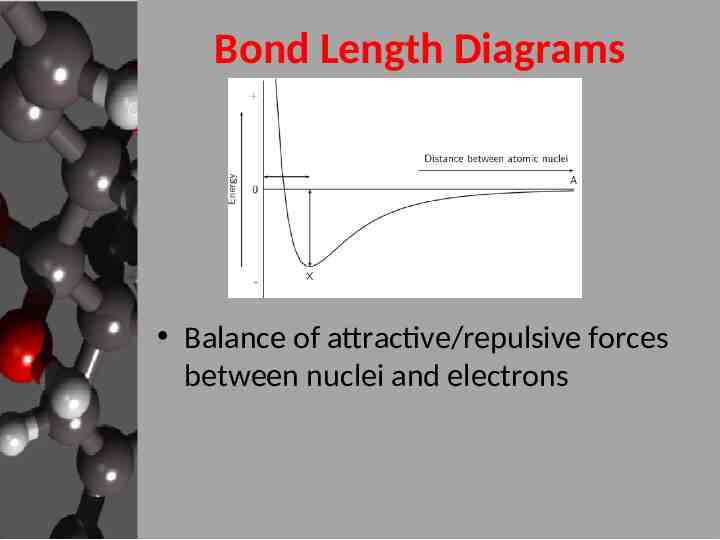
Bond Length Diagrams Balance of attractive/repulsive forces between nuclei and electrons
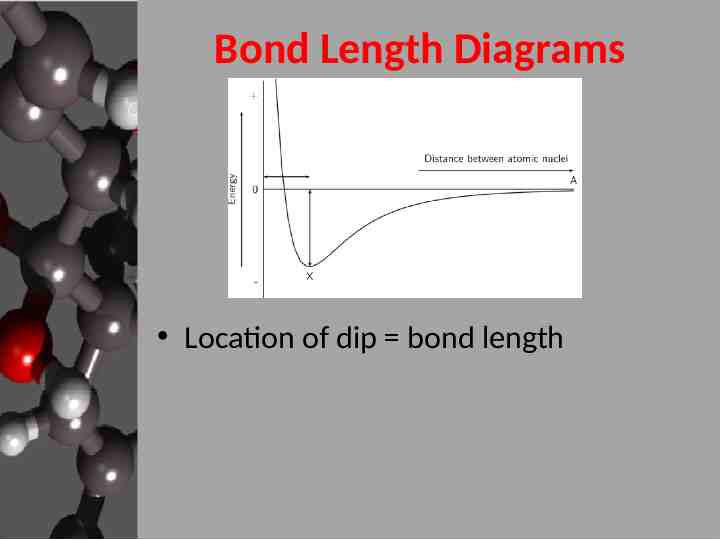
Bond Length Diagrams Location of dip bond length
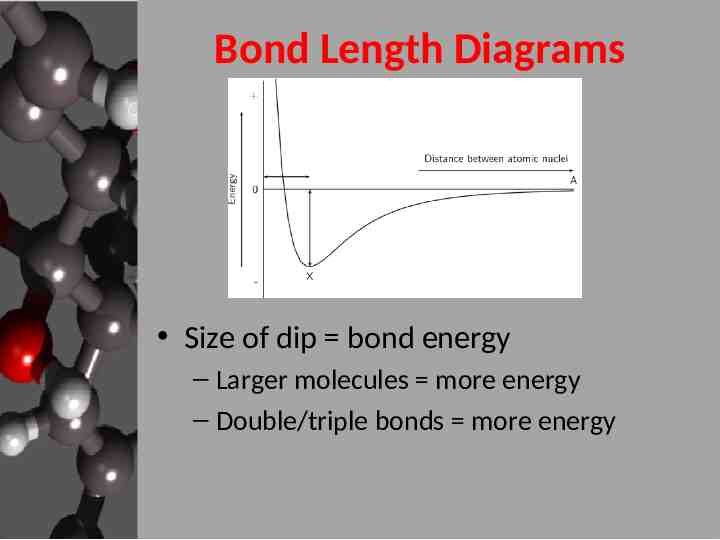
Bond Length Diagrams Size of dip bond energy – Larger molecules more energy – Double/triple bonds more energy

More Covalent Bonding Nonpolar symmetrical, equal pull in every direction Polar not symmetrical, greater pull in one direction Polar and nonpolar don’t mix

More Covalent Bonding Types of covalent bonds (rubber bands) – Single bond One shared pair of electrons Sigma bond Longest bond length Lowest bond energy Two shared pairs of electrons Sigma bond pi bond Shorter bond length than single Higher bond energy than single (less stable) Three shared pairs of electrons Sigma bond 2 pi bonds Shortest bond length Highest bond energy – Double bond – Triple bond

More Covalent Bonding LOTS more to come with drawing and what the shape of each of these molecules is Stay tuned .
Lewis Structures G.N. Lewis (1916) – Used lines and dots to represent bonding and valence electrons – A line was a covalent bond (2 e-) – A dot represents each valence electron
Lewis Structures Atoms – The number of dots is equal to the number of valence electrons in an atom – Fill each side of the symbol before pairing any electrons (except H and He) Examples:
Lewis Structures Ionic Compounds 1. Draw symbol for each atom 2. Transfer electrons until each metal has 0 valence electrons and each nonmetal has 8 valence electrons 3. Add multiple atoms of each type if necessary
Lewis Structures Ionic Compounds – Examples






