Advance Beneficiary Notice of Noncoverage (ABN) By: Valerie Hieb BS,
15 Slides291.24 KB

Advance Beneficiary Notice of Noncoverage (ABN) By: Valerie Hieb BS, MT (ASCP) Regulatory Manager – Sanford Laboratories

Advance Beneficiary Notice of Noncoverage What is an ABN? An Advance Beneficiary Notice of Noncoverage or ABN, is an acknowledgment by the Medicare beneficiary that he/she has been notified that the services provided may be deemed as not medically necessary by Medicare and that he/she is responsible for payment if Medicare denies payment. CMS Form: CMS-R-131 (Exp.03/2020) Services that Medicare reviews are called National Coverage Determinations (NCDs) and Local Coverage Determinations (LCDs). NCDs and LCDs provide guidance for administering the ABN form. Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage When should a notice be administered? An ABN must be administered anytime a provider orders services which Medicare may not cover. The ABN must be administered prior to specimen collection or before services are provided. Medicare may not cover testing for the following reasons: – Does not pay for the test(s) for the patient’s condition – The frequency limit for a test is exceeded – Experimental or research use tests Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage Why is the ABN administered? A properly administered ABN form protects the provider’s right to collect payment from the beneficiary when claims are denied by Medicare as “not reasonable and necessary.” Informs the Medicare beneficiary of the test(s) ordered and the estimated cost of those tests. If the ABN form is not completed properly, Medicare nor the beneficiary can be held responsible for payment. Sanford Laboratories Updated 5/29/2019
Advance Beneficiary Notice of Noncoverage All areas of an ABN form must be completed prior to specimen collection and before services are provided for the ABN to be considered valid by Medicare. How is an ABN administered? Failure to accurately provide all of the required information will result in an invalid ABN form. Medicare nor the beneficiary can be held responsible for payment if the ABN form is invalid. Sanford Laboratories Updated 5/29/2019
Advance Beneficiary Notice of Noncoverage A list of applicable NCDs and LCDs are available on the Sanford Laboratories website: www.laboratories.sanfordhealth.org Click on “Compliance” and scroll down to the “Printable Compliance Forms” section of the page and click on the appropriate link. Step 1: Determine if the test is medically Routine and screening tests are excluded by reviewed statute. An ABN is not required when a routine or screening diagnosis code is associated. Refer to the first section of the NCD PDF which provides a list of codes that are never covered by Medicare. Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage The Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) is updated by the Centers for Medicare and Medicaid Services (CMS) four times a year on the following dates: January 1st April 1st July 1st October 1st Local Coverage Determinations (LCDs) are added or updated periodically throughout the year by our Medicare Administrative Contractor (MAC). Sanford Laboratories Updated 5/29/2019

Medically Reviewed Tests– Medicare Part B National Coverage Determinations (NCDs) Urine Culture, Bacterial HIV (Prognosis Including Monitoring) HIV (Diagnosis) Blood Counts Partial Thromboplastin Time (PTT) Prothrombin Time (PT) Serum Iron Studies Collagen Crosslinks, Any Method Blood Glucose Testing Hemoglobin A1C/Glycated Protein Thyroid Testing Lipids Testing Digoxin Therapeutic Drug Assay Alpha-fetoprotein (AFP) Carcinoembryonic Antigen (CEA) Human Chorionic Gonadotropin (HCG) CA 125 CA 15-3, CA 27.29 CA 19-9 Prostate Specific Antigen (PSA) Gamma Glutamyl Transferase (GGT) Hepatitis Panel/Acute Hepatitis Panel Fecal Occult Blood Test Sanford Laboratories Updated 5/29/2019

Medically Reviewed Tests– Medicare Part B Local Coverage Determinations (LCDs) . Non-Covered Services Bladder/Urothelial Tumor Markers (UroVysion Test) B-Type Natriuretic Peptide (BNP) Testing Coenzyme Q10 (CoQ10) Controlled Substance Monitoring & Drugs of Abuse Testing Flow Cytometry Helicobacter Pylori Infection Testing Magnesium, Serum Measurement of Salivary Hormones MolDX: APC and MUTYH Gene Testing MolDX: Biomarkers in Cardiovascular Risk Assessment MolDX: BRCA/BRCA2 Genetic Testing Sanford Laboratories Updated 5/29/2019

Medically Reviewed Tests– Medicare Part B Local Coverage Determinations (LCDs) continued MolDX: CYP2C19, CYP2D6, CYP2C9, VKORC1 Genetics MolDX: Cystatin C Measurement MolDX: Foodborne Gastrointestinal Identified by Multiplex Nucleic Acid MolDX: Genetic Testing for BCR-ABL Negative Myeloproliferative Disease MolDX: Genetic Testing for Hypercoagulability/Thrombophilia (Factor V Leiden, Factor II Prothrombin and MTHFR) MolDX: Genetic Testing for Lynch Syndrome MolDX: HLA-B*15:02 Genetic Testing MolDX: HLA-DQB1*06:02 Testing for Narcolepsy MolDX: MDS FISH MolDX: MGMT Promotor Methylation Analysis MolDX: Molecular Diagnostic Tests (MDT) Sanford Laboratories Updated 5/29/2019

Medically Reviewed Tests– Medicare Part B Local Coverage Determinations (LCDs) continued MolDX: Molecular RBC Phenotyping MolDX: Multiplex Nucleic Acid Amplified Tests for Respiratory Viral Panels MolDX: NRAS Genetic Testing MolDX: Prometheus IBD sgi Diagnostic Policy Vitamin D Assay Testing This list may not be comprehensive as new LCDs are added periodically throughout the year. Please see the Sanford Laboratories website for the most up-to-date information. Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage Step 2: Determine if the diagnosis code is covered If the test(s) ordered are medically reviewed by Medicare, i.e. a NCD or LCD applies, and the diagnosis code (ICD-10CM) is not excluded based on statute, determine if the diagnosis code is covered. – If the diagnosis code is covered, an ABN is not required. – If the diagnosis code is not covered, an ABN form must be completed. Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage Step 3: Completing the ABN Form CMS-R-131 (Exp. 3/2020) must be used. Earlier forms are considered invalid. The form number is located on the bottom left hand corner of the ABN form. Detailed instructions for completing an ABN are available on the Sanford Laboratories website. Click on “Compliance” and scroll down to the “Printable Compliance Forms” section and click on the “Advance Beneficiary Notice of Noncoverage” link to locate instructions for completing an ABN. Sanford Laboratories Updated 5/29/2019

Advance Beneficiary Notice of Noncoverage Step 3: Completing the ABN Required Items –Notifier (Lab name, address and phone number) –Medicare Beneficiary’s Full Name –Name of lab test(s) that require an ABN –Reason Medicare may not pay (Check only one box) –Estimated cost The “Patient Fees to Use with ABNs” document is located on the Sanford Laboratories website. This document lists the cost for the most commonly ordered tests performed by Sanford Laboratories. If the test ordered is not on this document contact the Sanford Laboratories Accounts Receivable department at 605-328-5485 for pricing information. –The Medicare beneficiary or the beneficiary’s representative must choose only one option. –The Medicare beneficiary or the beneficiary’s representative must sign the ABN form. –The Medicare beneficiary or beneficiary’s representative must date the ABN form. Sanford Laboratories Updated 5/29/2019
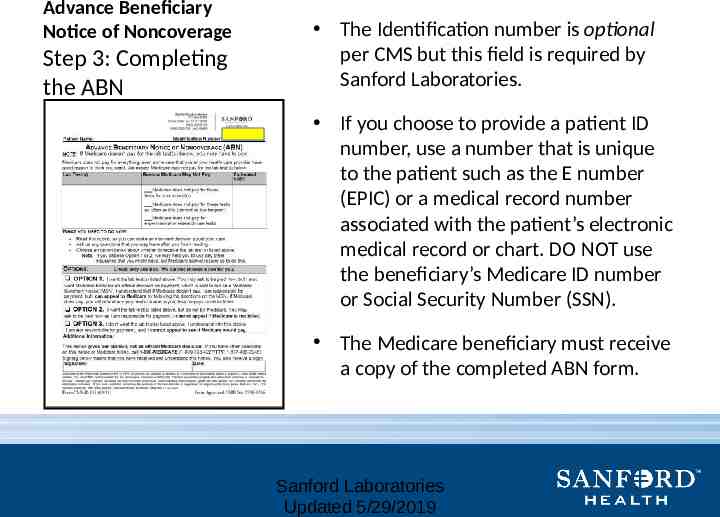
Advance Beneficiary Notice of Noncoverage Step 3: Completing the ABN The Identification number is optional per CMS but this field is required by Sanford Laboratories. If you choose to provide a patient ID number, use a number that is unique to the patient such as the E number (EPIC) or a medical record number associated with the patient’s electronic medical record or chart. DO NOT use the beneficiary’s Medicare ID number or Social Security Number (SSN). The Medicare beneficiary must receive a copy of the completed ABN form. Sanford Laboratories Updated 5/29/2019






