Chapter 14 Review Representative Problems
11 Slides1.36 MB

Chapter 14 Review Representative Problems
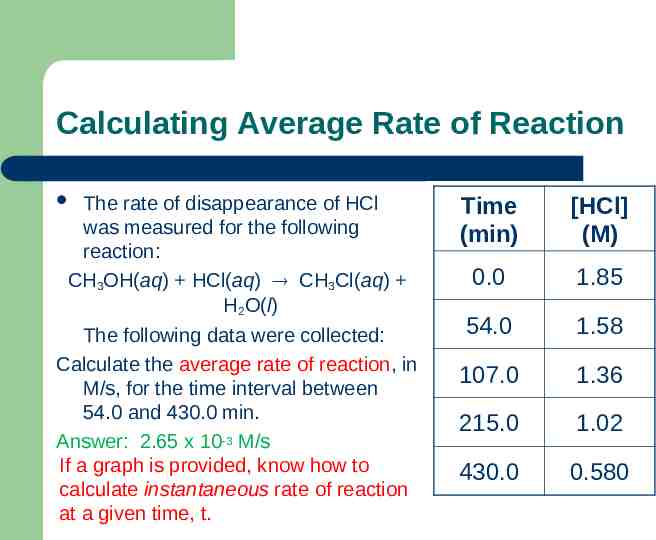
Calculating Average Rate of Reaction The rate of disappearance of HCl was measured for the following reaction: CH3OH(aq) HCl(aq) CH3Cl(aq) H2O(l) The following data were collected: Calculate the average rate of reaction, in M/s, for the time interval between 54.0 and 430.0 min. Answer: 2.65 x 10-3 M/s If a graph is provided, know how to calculate instantaneous rate of reaction at a given time, t. Time (min) [HCl] (M) 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580
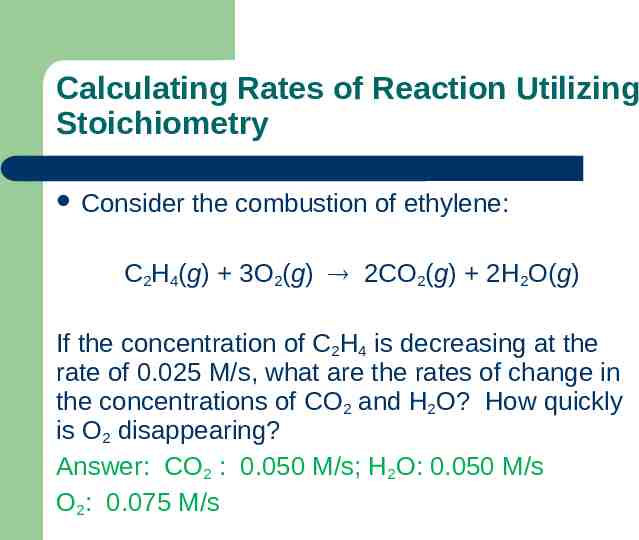
Calculating Rates of Reaction Utilizing Stoichiometry Consider the combustion of ethylene: C2H4(g) 3O2(g) 2CO2(g) 2H2O(g) If the concentration of C2H4 is decreasing at the rate of 0.025 M/s, what are the rates of change in the concentrations of CO2 and H2O? How quickly is O2 disappearing? Answer: CO2 : 0.050 M/s; H2O: 0.050 M/s O2: 0.075 M/s
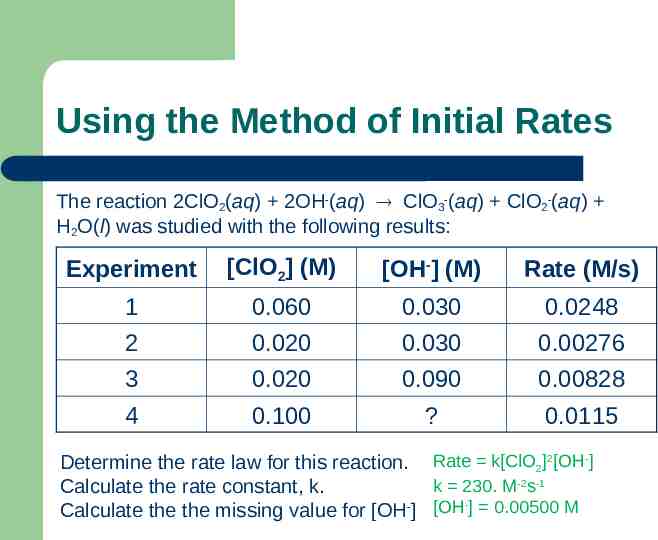
Using the Method of Initial Rates The reaction 2ClO2(aq) 2OH-(aq) ClO3-(aq) ClO2-(aq) H2O(l) was studied with the following results: Experiment [ClO2] (M) [OH-] (M) Rate (M/s) 1 0.060 0.030 0.0248 2 0.020 0.030 0.00276 3 0.020 0.090 0.00828 4 0.100 ? 0.0115 Determine the rate law for this reaction. Rate k[ClO2]2[OH-] k 230. M-2s-1 Calculate the rate constant, k. Calculate the the missing value for [OH-] [OH-] 0.00500 M
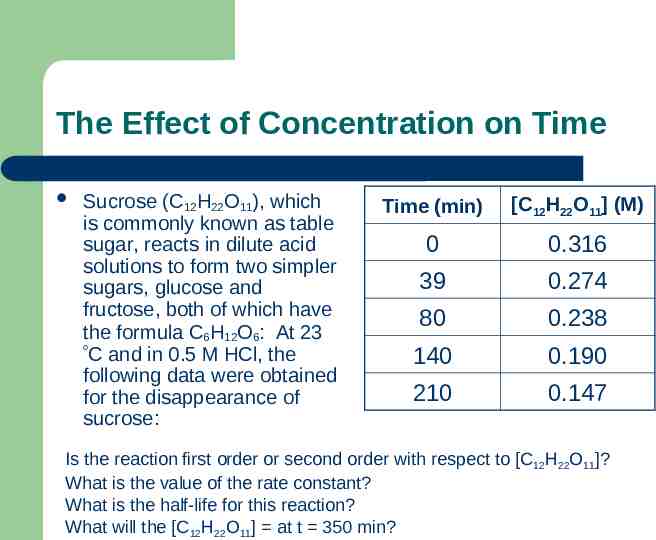
The Effect of Concentration on Time Sucrose (C12H22O11), which is commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6: At 23 C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) [C12H22O11] (M) 0 0.316 39 0.274 80 0.238 140 0.190 210 0.147 Is the reaction first order or second order with respect to [C12H22O11]? What is the value of the rate constant? What is the half-life for this reaction? What will the [C12H22O11] at t 350 min?
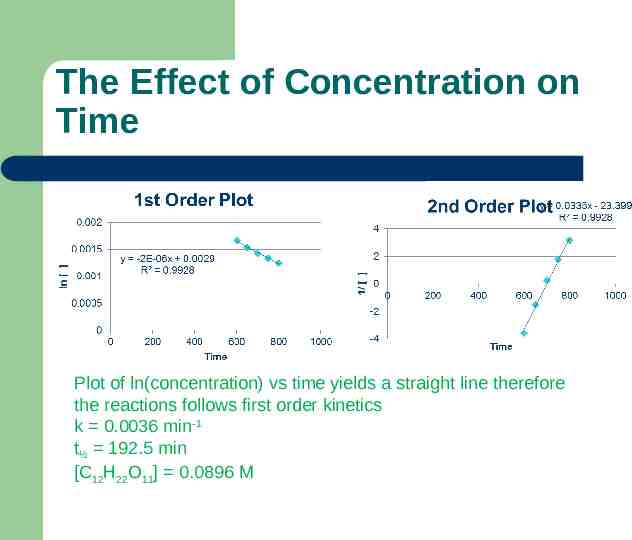
The Effect of Concentration on Time Plot of ln(concentration) vs time yields a straight line therefore the reactions follows first order kinetics k 0.0036 min-1 t½ 192.5 min [C12H22O11] 0.0896 M
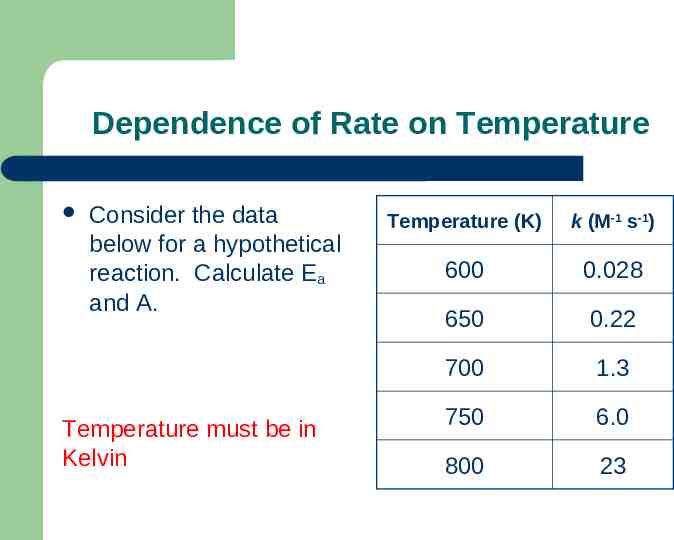
Dependence of Rate on Temperature Consider the data below for a hypothetical reaction. Calculate Ea and A. Temperature must be in Kelvin Temperature (K) k (M-1 s-1) 600 0.028 650 0.22 700 1.3 750 6.0 800 23
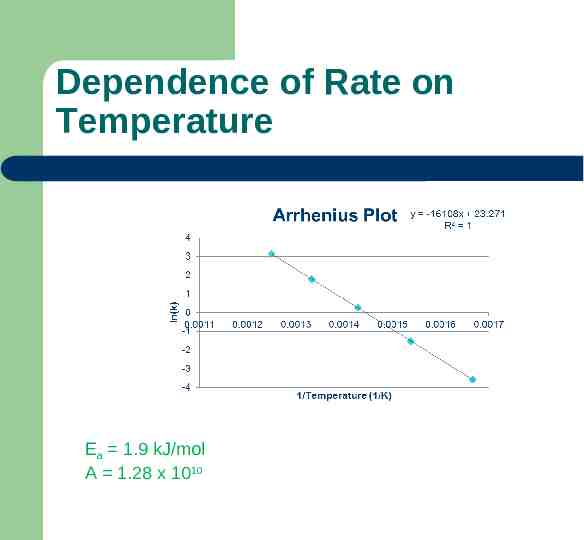
Dependence of Rate on Temperature Ea 1.9 kJ/mol A 1.28 x 1010
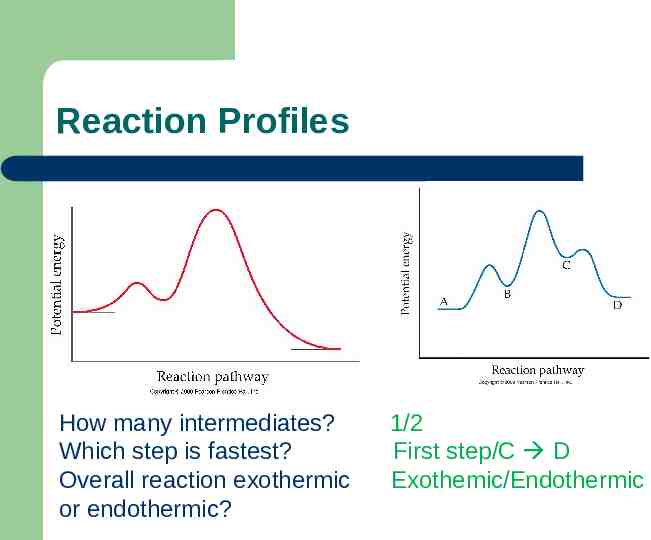
Reaction Profiles How many intermediates? Which step is fastest? Overall reaction exothermic or endothermic? 1/2 First step/C D Exothemic/Endothermic
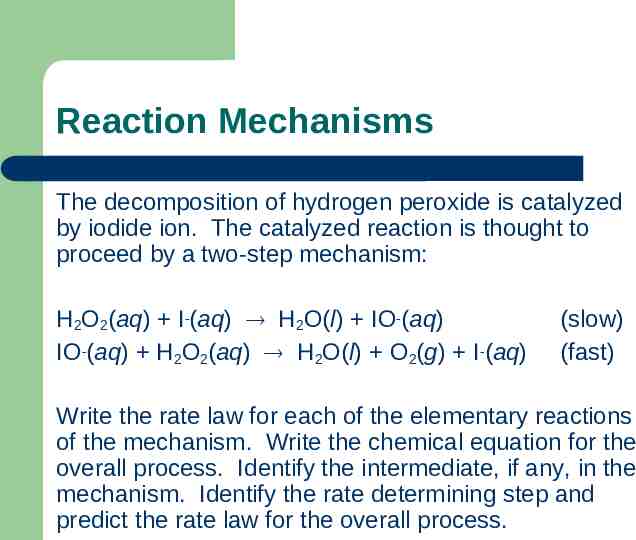
Reaction Mechanisms The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism: H2O2(aq) I-(aq) H2O(l) IO-(aq) IO-(aq) H2O2(aq) H2O(l) O2(g) I-(aq) (slow) (fast) Write the rate law for each of the elementary reactions of the mechanism. Write the chemical equation for the overall process. Identify the intermediate, if any, in the mechanism. Identify the rate determining step and predict the rate law for the overall process.
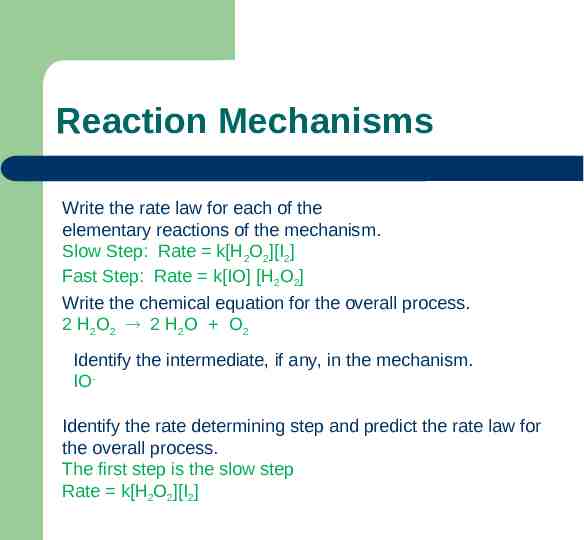
Reaction Mechanisms Write the rate law for each of the elementary reactions of the mechanism. Slow Step: Rate k[H2O2][I2] Fast Step: Rate k[IO] [H2O2] Write the chemical equation for the overall process. 2 H2O2 2 H2O O2 Identify the intermediate, if any, in the mechanism. IOIdentify the rate determining step and predict the rate law for the overall process. The first step is the slow step Rate k[H2O2][I2]






