Unit 04 Chemical Bonding
44 Slides1.04 MB

Unit 04 Chemical Bonding
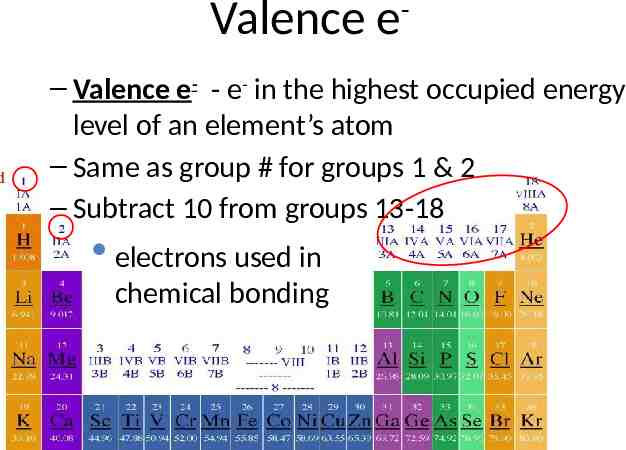
Valence e - – Valence e- - e- in the highest occupied energy level of an element’s atom – Same as group # for groups 1 & 2 – Subtract 10 from groups 13-18 electrons used in chemical bonding

Valence eLewis Dot Structure – shows number of valence e- Ex: bromine Br

White Board Practice K O C Ar

Learning Check Which Lewis Dot Structure is incorrect? A. C. Mg B. S B D. Xe

Octet Rule: - when forming compounds atoms want to have 8 e- (s2p6) like the noble gases (except He)

Cations (metals): positively charged ion, tend to lose elose 1 e 1s22s22p63s1 2 2 6 Na Na (atom) (cation) Using the Dot Structure octet lose 1 e- Na Na (cation) (neutral atom) Mg lose 2 e - Mg 2 (cation) (neutral atom) B 1s 2s 2p lose 3 e (neutral atom) B (cation) 3

Cations ca ion 1 2 3

Anions (Nonmetals) – negatively charged ions, gain e- Cl 1s 2s 2p 3s 3p 2 2 6 (atom) 2 5 gain 1 e- Cl octet Using the Dot Structure Cl gain 1e- O N (neutral atom) - (chloride ion) gain 2e- O 2- (neutral atom) Cl (neutral atom) 1s22s22p63s23p6 (chloride ion) valence - (oxide ion) gain 3e- N Nitride ion) -3

Anions -3 -2 -1

White Board Practice N gain 3e- N 3- (nitride ion) Lose 1 e- Li I gain e - Li I (chloride ion) Ca Lose 2e- Ca 2

Ionic Compound: Composed of a metal (cation) and nonmetal (anion) Ionic bond – oppositely charged ions attract Electrically neutral ( ) (-) Generally called salts Properties 1. ionic compounds form crystals 2. high melting and boiling points 3. hard and brittle 4. conduct electricity when dissolved in water or melted

Ionic Bonding 1 -1 1. Sodium and Chlorine donates e- Na Cl K K - Cl NaCl Does O have 8? dona tes e - Na 1 -2 2. Potassium and Oxygen O K donates e- O K K2 O -2

3 -1 3. Aluminum and Bromine Does Al have more electrons? Al Br 2 -3 4. Magnesium and Nitrogen Al Br - Br - Br - Does N have 8 yet? N Does N have 8 yet? Mg 2 N -3 3 Mg 2 N 2 Mg What about Mg’s other e-? Mg Mg Br Br Mg AlBr3 N Mg3N2 -3

Ionic Bonding – Title left side of spiral Ionic Bonding – Copy the problem – Draw the Lewis Structure for ea. element used in bonding – CATION RED – ANION BLUE/GREENISH – Show donated e- with an arrow – Show New ions charges – Put a BOX around the Formula

EXAMPLE: 1. Potassium & Fluorine K F K F KF -

Bonding in Metals Metallic bonds – the attraction between positive ions and surrounding mobile electrons Metal cations Sea of Electrons Good conductors of electrical current, ductile (wires), malleable (forced into shapes)

Covalent Compounds Also called MOLECULAR COMPOUNDS Formed from 2 or more nonmetals Low melting and boiling point

Important differences between covalent and ionic compounds Covalent compound Molecular compound Ionic compound Representative unit Molecule Formula unit (balance of oppositely charged ions) Type of elements Nonmetallic Metallic combined with nonmetallic Melting & Boiling point Low High Characteristic

Covalent Bonds – Forms when 2 atoms share a pair of valence e- Types of Covalent Bonds 1. Single Covalent Bond – two atoms share a pair of eEx: F2 Unshared pair – e- not shared between atoms F F F F and F F What makes this bonding work? Atoms have 8 e- in their outer level to make them stable

Covalent Bonds Ex: H2 H H H H Why does H2 only need 2 e- to be stable? first energy level only contains 2 e-

Covalent Bonds 2. Double Covalent Bond – 2 pairs of e- are shared between atoms Ex: O2 O O O O and O O

Covalent Bonds 3. Triple Covalent Bond – 3 pairs of e- are shared between atoms Ex: N2 N N N N and N N

Covalent Lewis Dot Structures 1. Draw each atom’s Lewis Structure 2. Connect single electrons until each atom has 8 e- (H only 2) 3. The central atom is often the first atom written & is usually the atom w/the least # of e-. (Exception – H can’t be the central atom)

Covalent Lewis Dot Structures 4. Place the unshared pairs around the atoms so ea. is stable (8 around it, except H – only 2) Examples: 1. Br2 Br Br Br Br

2. NH3 H N H N H H H H 3. CO2 O O C C O O

C Cl Cl Cl 5. H2O H O H O H H Cl C Cl Cl Cl Cl 4. CCl4

White Board Practice Problems: 1. CH4 C H H H H C H H H H
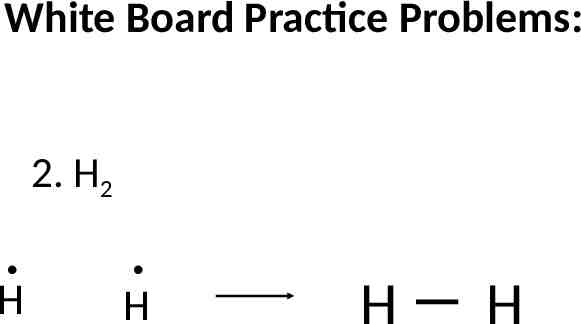
White Board Practice Problems: 2. H2 H H H H

White Board Practice Problems: 3. PH3 H P H H H P H H

White Board Practice Problems: 4. OF2 F F O F F O

White Board Practice Problems: 5. CHI3 H H I I C I I I I C

Title Left Side: Covalent Bonding Practice Draw the molecule then tell me how many double bonds does it have? 1. CO2 Draw the molecule then tell me what kind of covalent bond is formed. 2. P2 Draw the following molecules. 3. SiO2 4. CH4

VSEPR Theory Explains the shapes of molecules. The VSEPR theory states: b/c electrons repel each other, molecules adjust their shapes so that the valence e- pairs are as far apart from each other as possible. These are called electrostatic balanced positions

Shape Formula Bond Angle Electrons Linear AX2 180o 4 shared 0 unshared Linear AX 180o 1 shared 3 unshared Bent AX2 105o 2 shared 2 unshared Trigonal Pyramidal AX3 107o 3 shared 1 unshared Tetrahedral AX4 109.5o 4 shared 0 unshared Trigonal Planar AX3 120o 4 shared 0 unshared Contains a double bond

Bond Polarity Polar Covalent Bond – when 2 atoms are joined by a covalent bond and the bonding electrons are not shared equally

Bond Polarity Nonpolar Covalent Bond – when 2 atoms are joined by a covalent bond and the bonding electrons are shared equally

Differences between polar, nonpolar, and ionic bonds

How do you determine if a bond is polar, nonpolar, or ionic? Subtract the electronegativities of the bonding atoms (p. 265 in textbook)

Electronegativity Differences & Bond Type Type of Bond Electronegativity Difference Range Nonpolar Covalent Bond 0.0 – 0.4 Polar Covalent Bond 0.5 – 1.67 Ionic Bond greater than 1.67

Determine if the bonds between the following atoms are polar, nonpolar, or ionic: 1. Hydrogen and Carbon 2. Oxygen and Carbon 3. Potassium and Chlorine 4. Fluorine and Fluorine H 2.2 C 2.55 0.35Nonpolar O 3.44 C 2.55 0.89Polar K 0.82 Cl 3.16 2.34Ionic F 3.98 F 3.98 0.0 Nonpolar

Polarity of Molecule Polar Molecule – a molecule with a positive and negative end. Polar bonds must be present. Take into account if the molecule is symmetrical and if polar bonds are present.

Polarity of Molecule It is possible to have polar bonds but not a polar molecule! Carbon dioxide has 2 polar bonds and is linear (symmetrical). Bond polarities cancel out b/c they are in opposite directions. Oxygen Carbon Oxygen

Draw the dot structure of the following molecules – then predict the shape and polarity 1. I2 2. H2S 3. CHCl3 4. CO2






