Phase Diagrams Best, Chapter 14
22 Slides809.00 KB

Phase Diagrams Best, Chapter 14
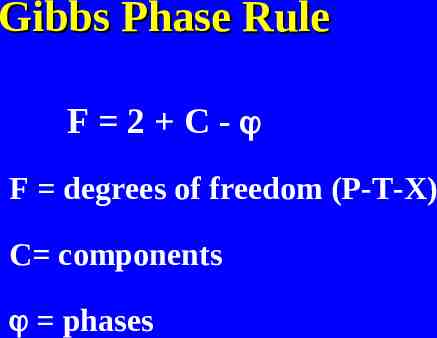
Gibbs Phase Rule F 2 C- F degrees of freedom (P-T-X) C components phases

Degrees of Freedom Rule applies to a phase or assemblage Divariant indicates two degrees of freedom Univariant means one degree of freedom Invariant means there are no degrees of freedom

Petrogenetic Grid The grid define stability limits – End-member minerals – Mineral assemblages More thermodynamic data is needed to construct a useful grid
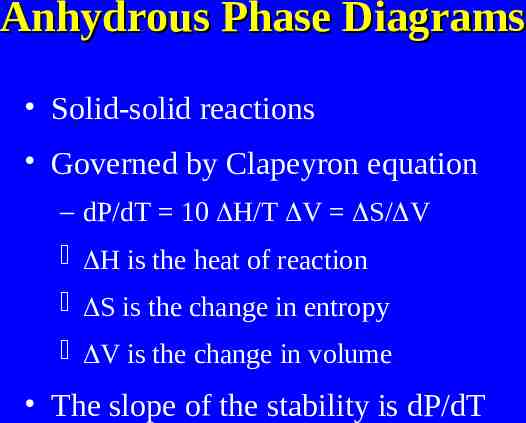
Anhydrous Phase Diagrams Solid-solid reactions Governed by Clapeyron equation – dP/dT 10 H/T V S/ V H is the heat of reaction S is the change in entropy V is the change in volume The slope of the stability is dP/dT
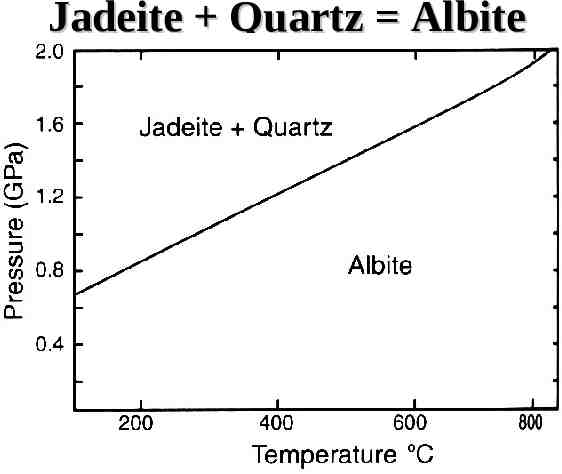
Jadeite Quartz Albite
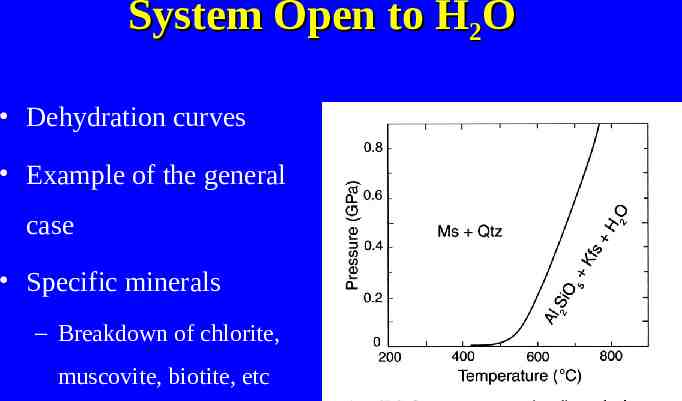
System Open to H2O Dehydration curves Example of the general case Specific minerals – Breakdown of chlorite, muscovite, biotite, etc
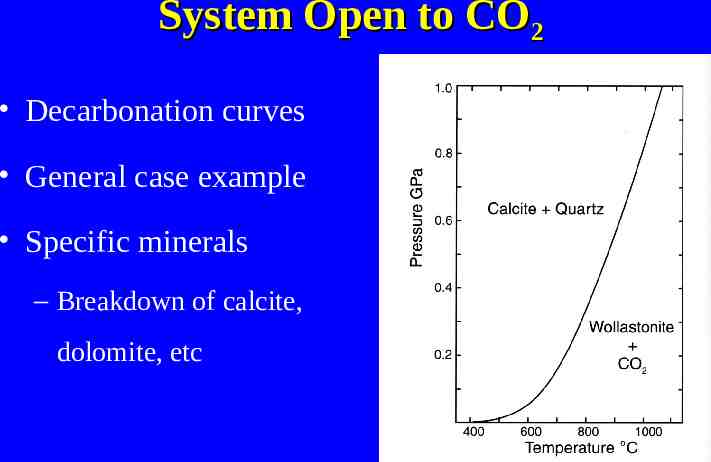
System Open to CO2 Decarbonation curves General case example Specific minerals – Breakdown of calcite, dolomite, etc
Univariant Curves Curves that define reactions with one degree of freedom In P-T space this means that if T is changed, than P must also change to maintain equilibrium Many important metamorphic reactions are defined by these curves
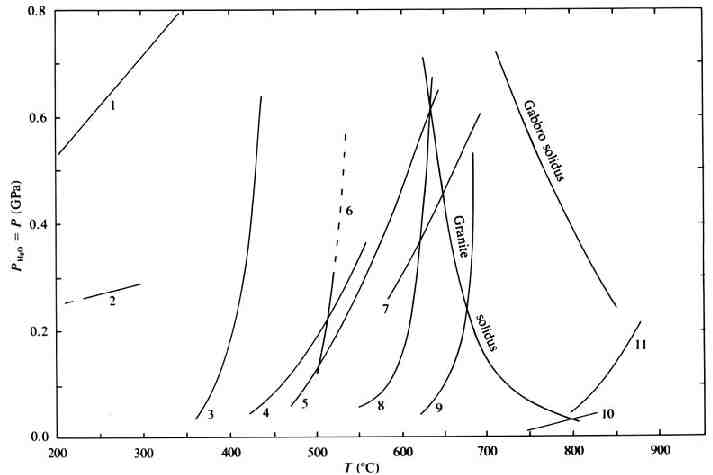
Important Reactions Al2O3 phase stability Dehydration curves
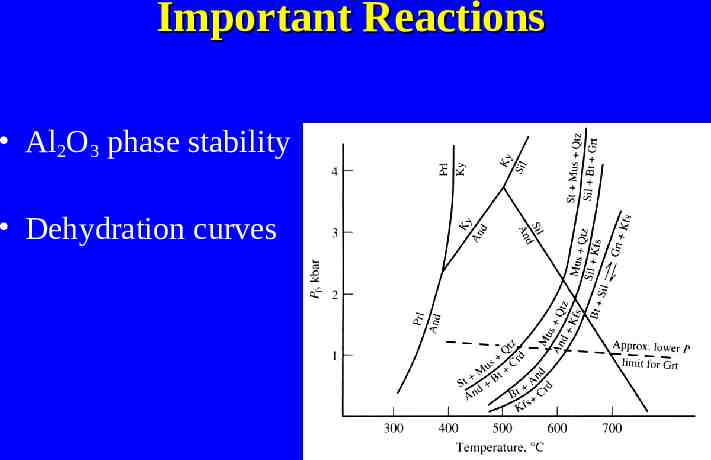
Stability of Iron Oxides PO2 (f O2 ) vs. Temp. Main phases – Hematite – Magnetite – Fayalite – Native Iron/Wustite
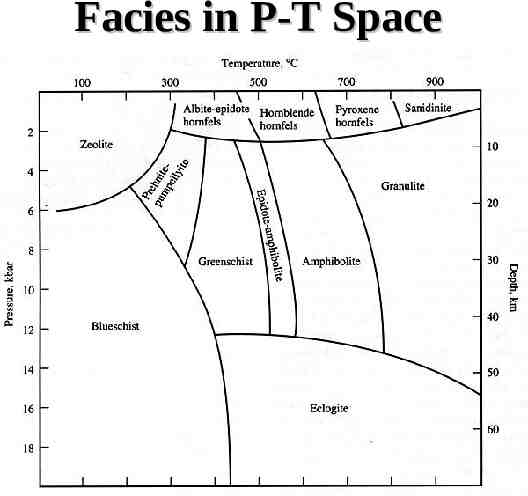
Miyashiro’s Facies Series Low geothermal gradient – Zeolite, pumpellyite-prehnite, blueschist Intermediate geothermal gradient – Barrow’s zones High geothermal gradient – Andalucite present in pelitic rocks

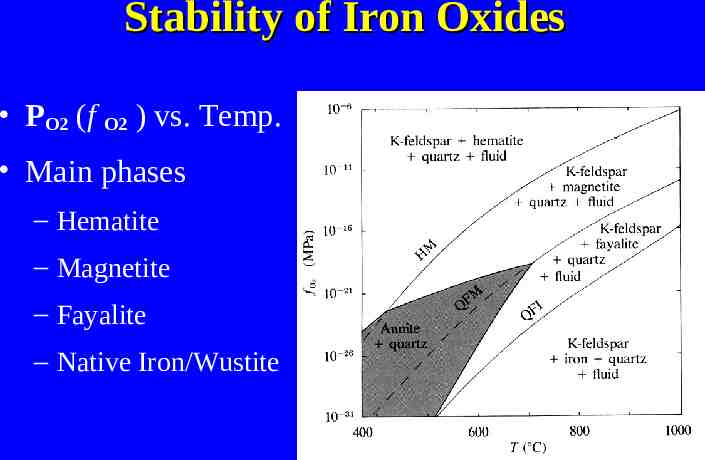
Relation to Geotherms High pressure series Medium P/T series High temperature series
Facies in P-T Space
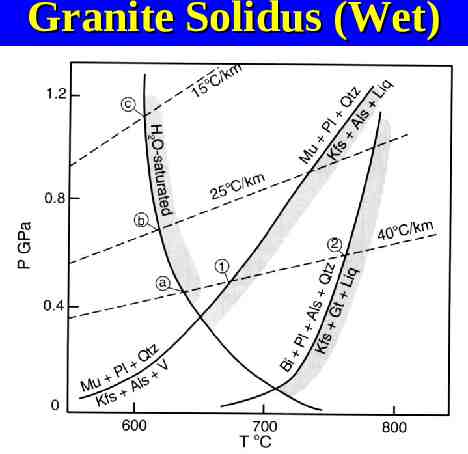
Granite Solidus (Wet)
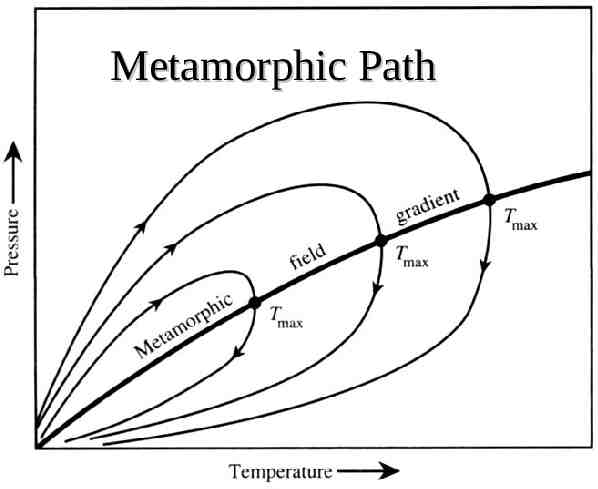
Metamorphic Path
Polymetamorphism Sometimes there are repeated episodes of metamorphism The last event may be weak or of short duration Polymetamorphism is common in post tectonic environments and in contact aureoles

Material Transport Diffusion Infiltration

Diffusion Materials move through crystal lattices or a stationary pore fluid Rate of movement controlled by a diffusion coefficient (Fick’s Law) Q k ( C/ x) Material moves about 1 cm/m.y.

Infiltration Passive mass transport of a solute in a moving fluid medium Driven by fluid pressure Microfractures are important Reaction-enhanced permeability – Volume reduction due to reactions Dilatency pumping

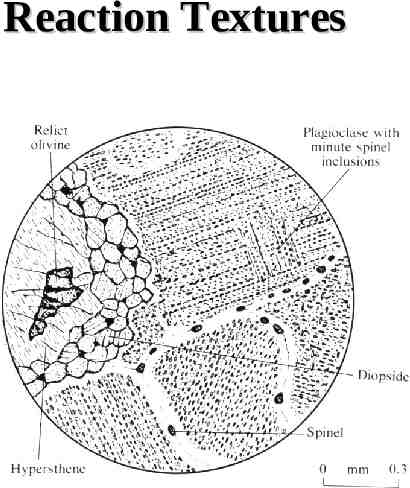
Reaction Textures olivine plagioclase hypersthene diopside spinel