Sudden Cardiac Death and Sport Dr Deirdre Ward Director, Centre for
38 Slides729.00 KB

Sudden Cardiac Death and Sport Dr Deirdre Ward Director, Centre for Cardiovascular Risk in Younger Persons Adelaide and Meath Hospital, Tallaght (and St James’, St Vincent’s University Hospitals) Blackrock Clinic and Charlemont Clinic

Centre for Cardiac Risk in Younger Persons (CRYP Centre) Service begins Jan 2007 Full-time, staffed Centre opens Nov 2008 All-day clinics, 1500 patients per year Nurse, 2 Technicians, Admin Officer, (Doctor) Funding Out-of-hours clinics, 600 patients Cardiac Risk in the Young Charity (CRY-Ireland) Adelaide Society Tallaght Hospital Volunteers Pfizer Private donations Continuous fundraising Tallaght Hospital and TCD Aim : provide timely, comprehensive assessment of families where SCD has occurred or young people with worrying cardiac symptoms Tests : ECG, Echo, Exercise Test, Heart rhythm monitor all on one day, followed by Consultation with Consultant (family tree etc)

Overview of Sudden Cardiac Death Size of the problem Causes of sudden cardiac death Sport and SCD Identifying those at risk Managing risk General screening ? Public access defibrillators ?

Background Sudden Cardiac Death death from definite or probable cardiac causes within 1 hour of symptom onset Incidence from International Studies 1 to 3 per 100,000 in those 1 to 35 yrs of age 10 to 75 per 100,000 in those 35 to 64 yrs Incidence in Ireland Extrapolation from other studies suggest approx 5,000 SCD annually RoI, 2000 NI 60 - 80 deaths 35 yrs (RoI), 25 (NI) From 2005 study of Coroners data 5 per 100,000 males (14-35 yrs) 1 per 100,000 females (14-35 yrs)

In context 134 drug-related deaths in Dublin in 2007 87 murder/manslaughters in State 2007 336 road deaths in 2007 82 pedestrians 138 drivers 70 passengers

Causes of SCD Over 35 yrs of age Coronary Heart Disease (‘hardening of the arteries’) Under 35 yrs Cardiomyopathies (heart muscle disorder) Congenital Heart Disease (‘hole in heart’, ‘blue baby’) ‘Structurally Normal Heart’ (ion channel disorders, conduction disease) SADS Anomalous coronaries (abnormal anatomical position of coronary blood vessels) Myocarditis (infection or inflammation of heart muscle)
Hypertrophic cardiomyopathy (HCM or HOCM) Increased thickness of heart muscle Most common inherited cardiac disease Prevalence 1 in 500 people carry gene 11000 in 32 counties 90% of cases thought to be inherited (runs in family) 10% ‘sporadic’ – pass on to their children? Approx 50% who inherit genetic change develop fullblown condition (‘incomplete penetrance’) Inheritance pattern Autosomal Dominant 50% risk of inheriting gene if parent affected

HCM Symptoms include : Shortness of breath with exercise chest pain (usually with exercise) Diziness (at rest or with exercise) blackouts Palpitations No symptoms Risk of sudden death 1% per year Intensive exercise can increase risk Usually identifiable on ECG and Echo
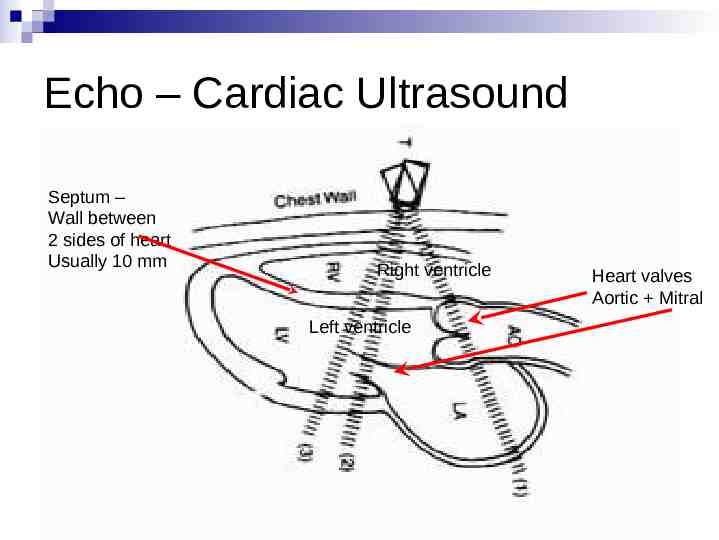
Echo – Cardiac Ultrasound Septum – Wall between 2 sides of heart Usually 10 mm Right ventricle Left ventricle Heart valves Aortic Mitral

HCM - Treatment No cure, but can prevent complications Manage symptoms Medications (Beta-blocker tablets) Modify lifestyle Surgery (only in very limited circumstances) Ensure family members checked Assess risk of sudden death Low-risk, reassure, but still avoid intense exercise High-risk, recommend implantable defibrillator (ICD)
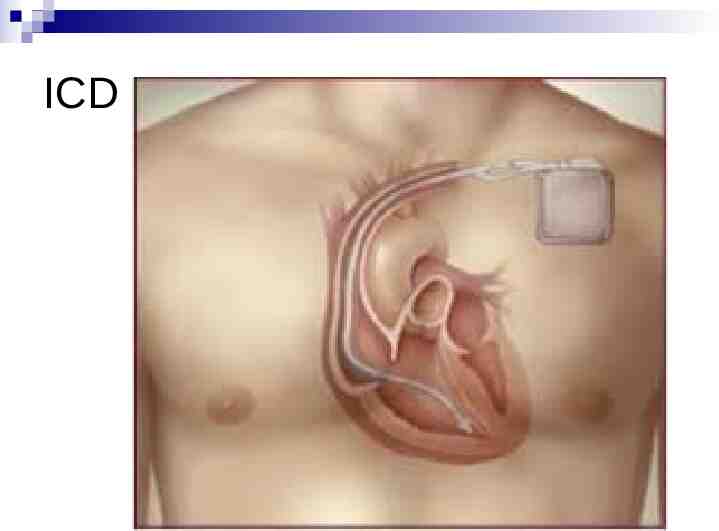
ICD
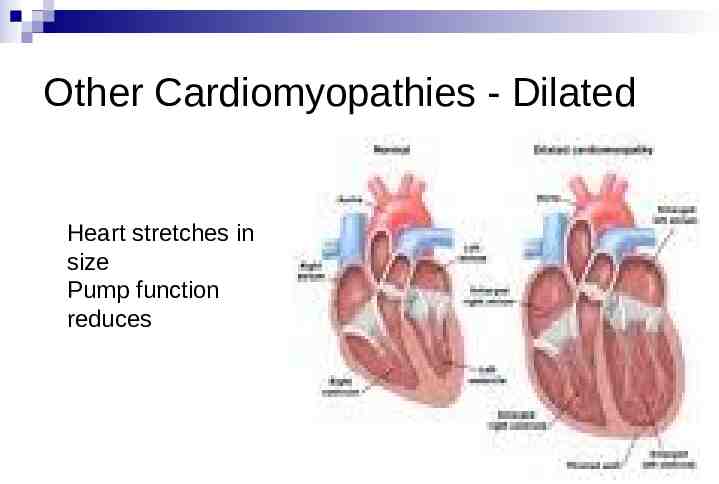
Other Cardiomyopathies - Dilated Heart stretches in size Pump function reduces

Other Cardiomyopathies- Dilated May be inherited, much less common 1000 people in country Other causes include viral illness, drugs, alcohol May cause shortness of breath, palpitations, blackout, sudden death ECG and echo usually identifies Other tests may be necessary Treatment Medications Occasionally pacemakers and/or ICD Risk of SCD usually highest in those with poorest pump function, who usually have symptoms
Other Cardiomyopathies – Arrhythmogenic (aka ARVC or ARVD) Heart may become enlarged Scarring develops in heart Causes palpitations, dizzy spells, blackouts, shortness of breath, sudden death Often inherited May need several tests to diagnose ECG, echo, Exercise test, heart rhythm monitor, MRI scan of heart Milder cases can be missed (even in Italy with compulsory screening programme) Treatment Medications Lifestyle modification If considered high risk of rhythm problems, recommend ICD

Other inherited conditions Marfan’s syndrome Congenital heart disease Weakness of walls or large blood vessels May be associated with tall stature and hyperflexibility, eye problems Identified on physical exam, echo and X-ray scans Abnormal development of cardiac structure(s) in the womb Range from ‘blue baby’ to small holes in heart Milder forms generally not life-threatening 10 % inherited, most occur spontaneously Mitral valve prolapse 1% of population have at least mild case Severe cases may be associated with sudden death May be over-estimated as cause of sudden death

Other conditions Valve disease Anomalous coronaries Usually causes a murmur May cause reduction in exercise tolerance Anatomical variant in placement of blood vessels Some may reduce blood supply during stress or exercise but most probably don’t cause problem and may be over-estimated as cause of SCD Myocarditis Inflammation of heart muscle Usually thought to follow viral infection 1/8 people with virus fever have ECG change Probably should avoid exercise during viral infection Possible genetic predisposition to being affected by virus

Sudden Arrhythmic (Adult) Death Syndrome (SADS) ‘Diagnosis of exclusion’ Sudden death occurs, and is consistent with cardiac rhythm disturbance, but post-mortem examination finds no abnormality Currently no standardization of post-mortem examination in Ireland (improving) Currently no Specialist Cardiac Pathologist with specific responsibility If post-mortem not carefully done Structural cause of death may be missed Minor abnormalities may be incorrectly recorded as cause of sudden death True number of SCD which are actually due to SADS probably underestimated Electrical problem is cause of death, but no electrical activity after death so not detectable at post-mortem

Electrical problems – also known as ‘Channelopathies’ Electricity in heart is generated by pump channels in walls of each cell in heart pump salts (Na, K, Ca) in and out of cell Pump channel ion channel If pump malfunctions (under or over-active) changes electrical activation of heart which causes electrical instability and increases chance of arrhythmia May not cause symptoms unless palpitations, dizzy episodes or blackouts Usually detectable on ECG (if looking for it) Different genes code for different pumps and mutations cause different conditions : Long QT syndrome Brugada Syndrome Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) Not identifiable on PM Can be identified on ECG ( /- exercise test and rhythm monitor) in living 40% of families of those who die of SADS have inherited cause identified (mostly LQT syndrome and Brugada syndrome)

Influence of sporting activity on risk In younger people over all, sporting activity increases risk x 2.5 Older adults who exercise frequently have 5x increased risk of sudden cardiac arrest during vigorous activity (coronary disease) Older adults who do not exercise frequently have 56 x risk of SCA during vigorous activity (NEJM 1984)

Sport and sudden cardiac death If you have one of these cardiac conditions intense sporting activity will double risk of dying suddenly (eg increase from 1% to 2% in HCM) You do NOT have to be an athlete to die from SCD You CAN die from SCD at rest or during sleep

Identifying those at risk Family history Symptoms Premature sudden deaths definitely or possibly cardiac Relatives diagnosed with above conditions SOB or chest pain that limit exercise Unexplained dizzy spells / blackouts (especially if on exertion) Prolonged palpitations ‘Screening’ Physical exam? ECG? Other?

Management of at risk people Not everyone with these conditions has high risk of sudden death Risk varies with each condition and even within families (the same gene will behave differently in everyone who inherits it) System for identifying at risk people developed in most conditions

Managing risk Avoid competitive sport or very strenuous exertion Recreational sport, PE classes etc usually safe Medications in some (eg b-blockers) Continued observation in all Implantable defibrillators in some Cost implications Complications

Why screen relatives, or people with suggestive symptoms? Many conditions relatively easy to identify (if you know what you’re looking for) Not everyone affected is at risk Varying success rates at accurately identifying at risk people Some can be treated with medication High risk people offered implantable defibrillator (ICD or ‘shock-box’) Future generations at risk

Cardiac evaluation for families or symptomatic individuals Current options GP evaluation Local physician General Cardiologist Specialist Centre Centre for Cardiac Risk in Younger Persons (Tallaght / St James / St Vincent’s) Family Heart Screening Clinic (Mater and Blanchardstown Hospitals)

Athlete / Population screening Currently no government resources for screening high-risk population Risk in general population approx 1to 3 per 100,000 athletes/yr Potential downside to ‘screening’ Sport can bring on changes in cardiac tests (espec ECG but also Echo) that may be difficult to distinguish from cardiomyopathy Additional testing in perhaps 10% of all those screened Borderline cases may never be resolved completely ? affect life insurance in future ? Restrict ability to play sport ? Restrict career choices

If considering Irish National programme Questions : Who would oversee (GP vs Cardiologist)? Who (athletes only or every person?), when (at what age?) and how often (repeated?) What form should it take? Who pays? Who deals with fall-out from abnormal results Voluntary or compulsory?

AHA Consensus Panel Recommendations For Pre-participation Screening Family History: 1. Premature sudden death 2. Heart disease in surviving relatives Personal History: 3. 4. 5. 6. 7. 8. Heart murmur Systemic hypertension Fatigability Syncope Exertional dyspnoea Exertional chest pain Physical examination: 9. Heart murmur (supine / sitting / standing) 10. Femoral pulses 11. Stigmata of Marfan Syndrome 12. Blood pressure measurement
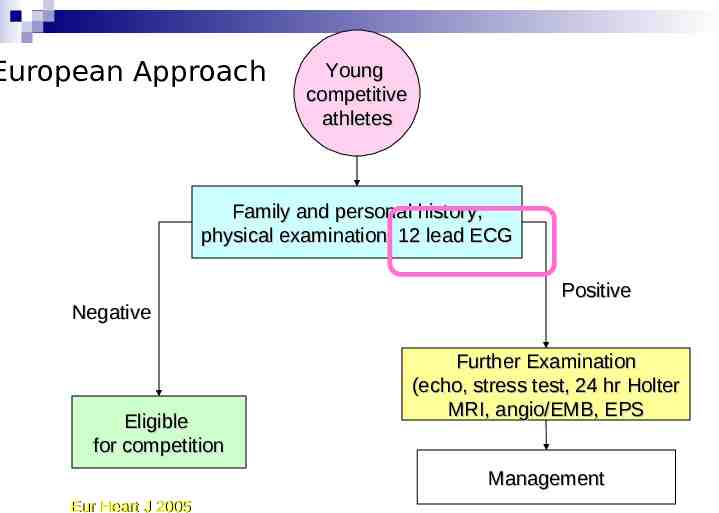
European Approach Young competitive athletes Family and personal history, physical examination, 12 lead ECG Negative Eligible for competition Positive Further Examination (echo, stress test, 24 hr Holter MRI, angio/EMB, EPS Management Eur Heart J 2005
Difficulties with screening Low prevalence diseases so prior probability low Questionnaire alone Physical examination Family history may not be known Conditions can occur without SCD Symptoms not recognised or suppressed Allows potential pick-up cardiac murmurs (HCM, bicuspid aortic valve, MVP) and coarctation, Marfan’s HCM may be present without murmur, misses other cardiomyopathies ECG Improves pick-up of cardiomyopathies, LQT etc Changes may be subtle Will not identify anomalous coronaries

Benefits of Italian programme (Corrado et al, JAMA 2006) Screening by law since 1982 Everyone 12 yrs of age or older engaged in formal competitive sport Repeated every 2 years Performed by ‘Sports Cardiologist’ Published review of athlete screening, and causes of SCD in athlete and non-athlete population in 2006 9% of athletes required further screening 2% of athletes disqualified
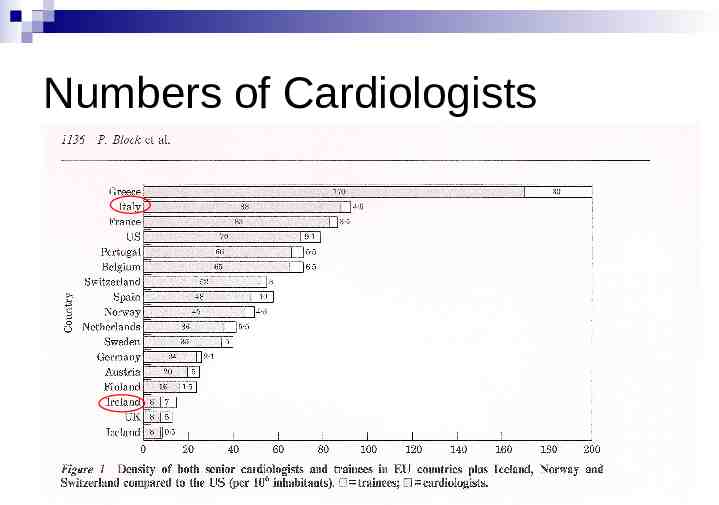
Numbers of Cardiologists

Automatic Defibrillators (AEDs) Prominent placement in public locations (? remote rural towns also) Computer analyses heart rhythm and decides if shock is required Ideally personnel using should be trained (and training updated ? every 3 months) Have been successfully used by untrained ‘good samaritans’ Maintenance issues Public liability (Duty of Care issues) If cardiac arrest during sport more difficult to resuscitate

Data from US ‘Schools’ 15 year period reviewed Number of schools needed to generate 1 cardiac arrest per year 167 schools 8 colleges / universities Of those who had cardiac arrest 15 % were 35 years of age 10% were students (half of them were already known to have health problems)

In Summary SCD is not common High-risk people usually identified by symptoms or family history – priority for evaluation Cure not possible, but correct management can prevent complications

Symptoms to be aware of Awareness of unusual symptoms important: Chest discomfort and/or Shortness of Breath that significantly limits ability to exercise Unexplained blackouts Prolonged palpitations (especially if associated with diziness)

Reducing the risk Identify those with underlying conditions Older people returning to sport get checked by GP Improve response in the event of a cardiac arrest Availability of AEDs Training of population in Basic Life Support Improved ambulance response times

Cardiac screening for sports or entire population? Hard to justify compulsory testing Ethical right not to know about health issues Currently no resources in public health system for statistically low-risk Privately funded facilities exist Beware variable standard of expertise and focus on profit