SQM Supplier Training D00851806 Revision A
58 Slides8.96 MB

SQM Supplier Training D00851806 Revision A

Chapters 1 What is SQM & Why? 5 Unique Situations 2 Supplier Expectations 6 Troubleshooting 3 Regulatory Agency Awareness 7 Resources 4 SQM System 8 Next Steps 2 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
What & Why

What is SQM? Supplier Quality Management SQM is a part of the Supplier Owned Quality (SOQ) umbrella that covers all supplier data provided to Medtronic SQM is a system that utilizes supplier data electronically uploaded and valuated against specific product requirements 4 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only SQM is an opportunity to improve the collaboration between Medtronic and its suppliers

Why SQM? Pre-determine lot acceptance and provides shipment authorization upon successful data upload. Provide real time feedback prior to release of product and identifies opportunities for improvement. Industry and Regulatory Agencies demand that we identify, monitor, and control any potential risks with our suppliers by working together. Ultimately, to provide the most reliable and robust products to end users, the Doctors and most importantly the Patients that we serve. 5 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Supplier Expectations
Expectations - Ownership Product Quality Integration Communication We are all responsible to ensure that all requirements are met per specifications and purchase order as necessary and is not limited to only the parameters being submitted through SQM. The SQM upload process should be part of process flow. Think collaboration! – we will work together to achieve our goals! Execute as part of site process or Medtronic Process located at https://sqm.medtronic.com/msq m/documents.html Confirm use of latest SQM template 7 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only Communicate any issues or concerns to the SQM team as soon as possible (utilize support mailbox [email protected])
Expectations - Compliance Process Control Traceability It is important that lot traceability be maintained. This is done by ensuring that the lot or batch identification matches the certification of compliance (CoC), Maintain test equipment to ensure SupplierLotID in SQM and any other they are capable of valid results. document with the shipped product. Control incoming materials, equipment, and processes to ensure products meet quality requirements. Any potentially nonconforming material is dispositioned timely. 8 Everything that is uploaded in SQM is traceable and can be searched by lot, product or date. SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only Regulatory Agencies SQM Uploads are electronic records and we need to ensure record integrity As it applies to SQM, compliance has to do with record integrity and management of user IDs and passwords. Each user will have their own user ID and password – not to be shared with others
Expectations – Data Integrity Data Violation Reaction Plan Ensure data is complete and accurate and that it successfully loads into the system without failure If data fails, follow internal quality system to determine why it failed and how to fix it Product meets specification limits, or other acceptance limits as defined in the Selected Product Controls (SPC) form If an unexplainable error occurs, reach out to SQM Help Desk for resolution ( [email protected]) Ship authorization will be determined by successful notification within the SQM system. Pre acceptance of the product must occur prior to shipment. 9 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Medtronic Password Policy Password Requirements At least 7 characters, but not more than 10 characters Uppercase characters/lowercase characters (or combinations) Numeric (0-9) characters No symbols Cannot contain a user’s account name Strongly recommended to not contain dictionary words 10 Implementation Compromised passwords known to others (or passwords suspected of being compromised) must be changed immediately. When logging on to an account for the first time, the account owner will be prompted to immediately change the temporary password and select a new password. The re-use of any of the last 10 passwords selected for a user account is not permitted. User account passwords expire and require changing every 180 days. In case of an employee leaving or being terminated please notify Medtronic and account will be disabled. SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

SQM System
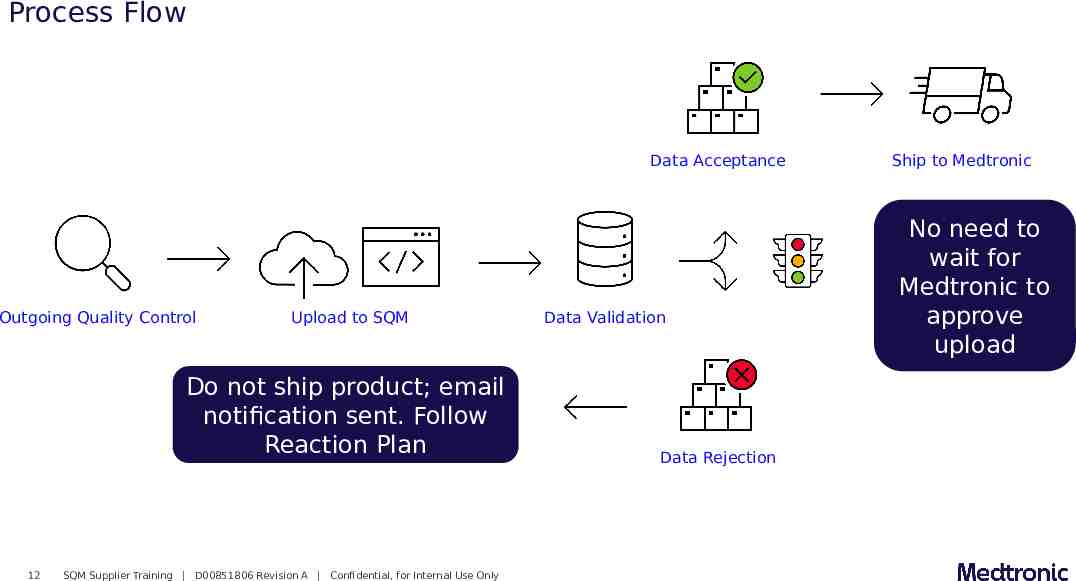
Process Flow Data Acceptance Outgoing Quality Control Upload to SQM Do not ship product; email notification sent. Follow Reaction Plan 12 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only Data Validation Data Rejection Ship to Medtronic No need to wait for Medtronic to approve upload

SQM Generator Template Template is available to help you create an XML file for data upload to the SQM system. ‘White’ cells require data input (unique lot identifiers or test data). You must use most current tool revision sent to your site. 13 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
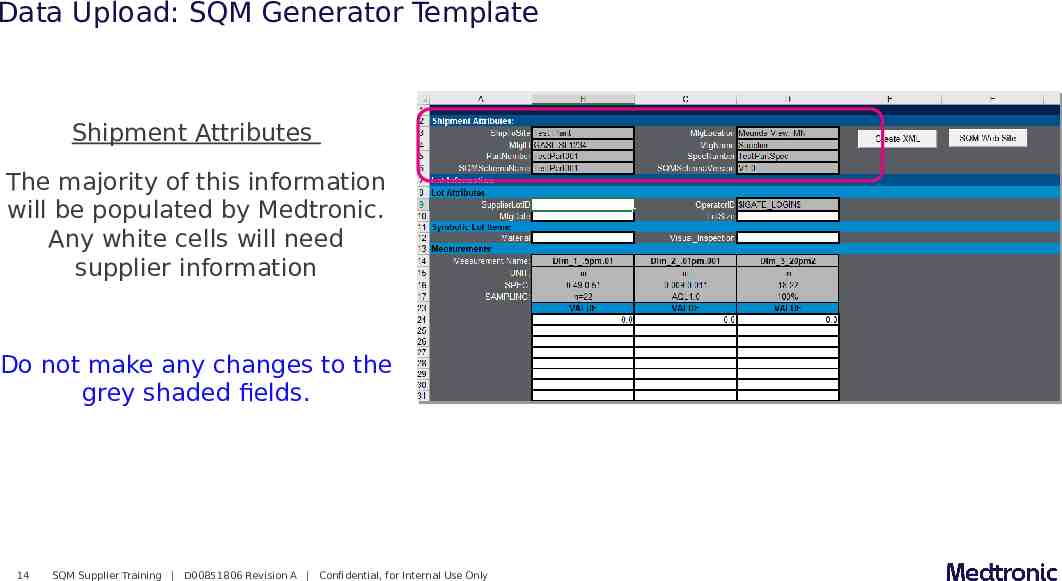
Data Upload: SQM Generator Template Shipment Attributes The majority of this information will be populated by Medtronic. Any white cells will need supplier information Do not make any changes to the grey shaded fields. 14 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
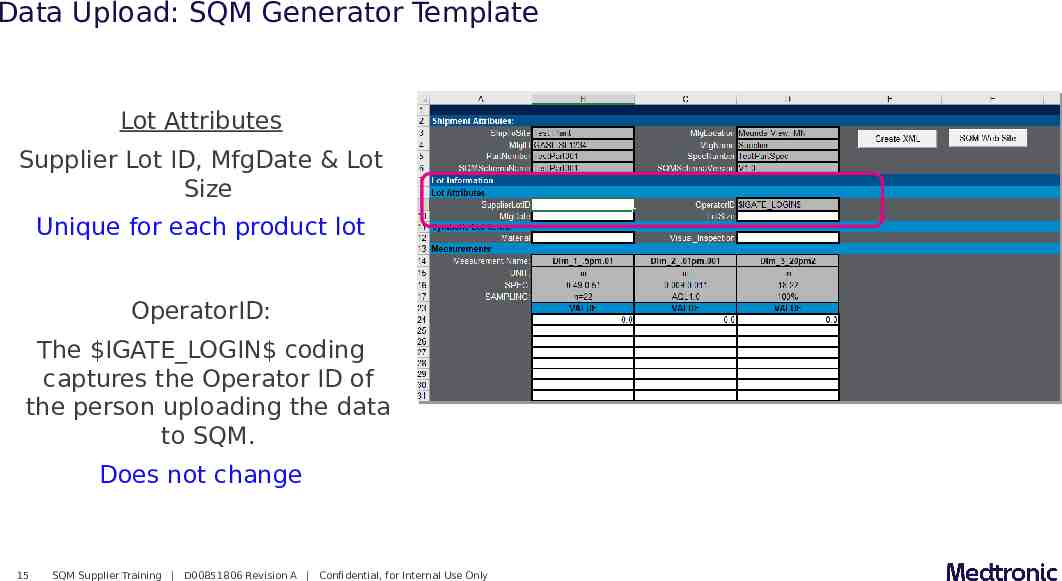
Data Upload: SQM Generator Template Lot Attributes Supplier Lot ID, MfgDate & Lot Size Unique for each product lot OperatorID: The IGATE LOGIN coding captures the Operator ID of the person uploading the data to SQM. Does not change 15 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Data Upload: SQM Generator Template Symbolic Lot Items: If symbolic items are required for lot acceptance, these will be pre-populated drop-down values (Attribute inspection i.e. visual inspection or Material confirmation). If symbolic items are not required, this section will not be included in the template. 16 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Data Upload: SQM Generator Template Measurements These columns are the variable data features to be submitted. For your convenience, the data unit (UNIT), spec limits (SPEC) and sampling requirements (SAMPLING) will be included. Please verify the data units in the template match the data output units from your test systems. 17 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
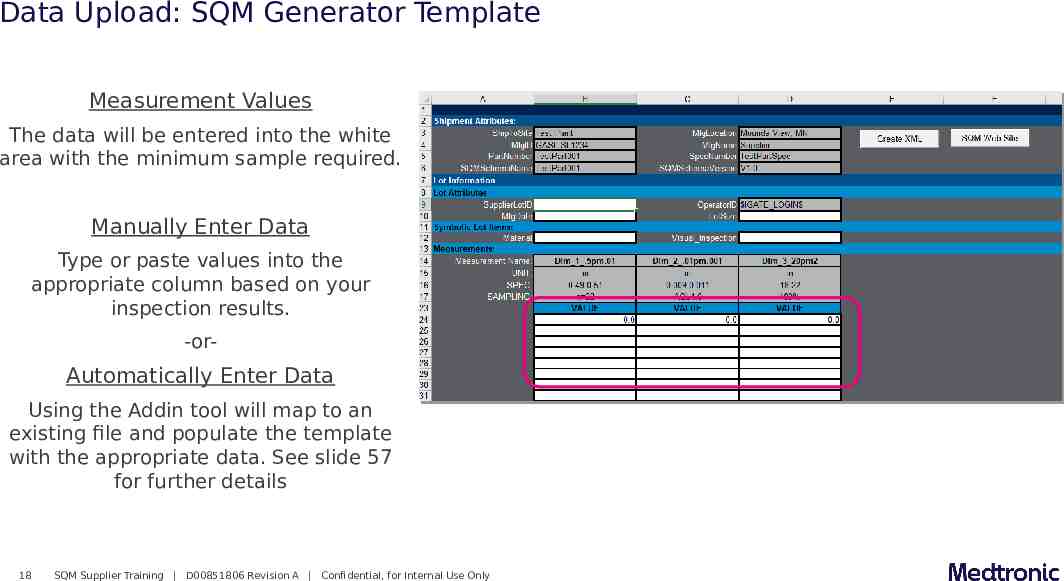
Data Upload: SQM Generator Template Measurement Values The data will be entered into the white area with the minimum sample required. Manually Enter Data Type or paste values into the appropriate column based on your inspection results. -or- Automatically Enter Data Using the Addin tool will map to an existing file and populate the template with the appropriate data. See slide 57 for further details 18 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
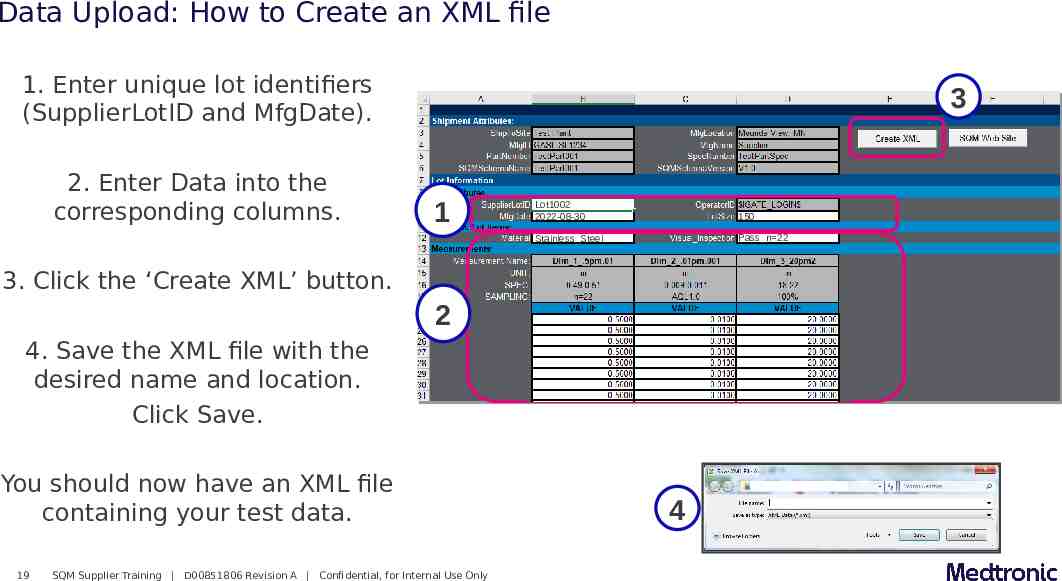
Data Upload: How to Create an XML file 1. Enter unique lot identifiers (SupplierLotID and MfgDate). 2. Enter Data into the corresponding columns. 3 1 Lot1002 2022-08-30 150 Stainless Steel Pass n 22 3. Click the ‘Create XML’ button. 2 4. Save the XML file with the desired name and location. Click Save. You should now have an XML file containing your test data. 19 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 4

Data Upload: XML File 1 The XML file will contain: 1. Header information (i.e. SupplierLotID, MfgDate, ShipToSite, MfgLocation, MfgID, PartNumber, SpecNumber). 2 3 2. Symbolic pass/fail (if applicable) 3. Variable data results 20 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Upload and Data Review Data can be submitted in the SQM system via: 1. Upload file through website 2. Secure FTP Chrome works best Access will be provided during training http://sqm.medtronic.com 21 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
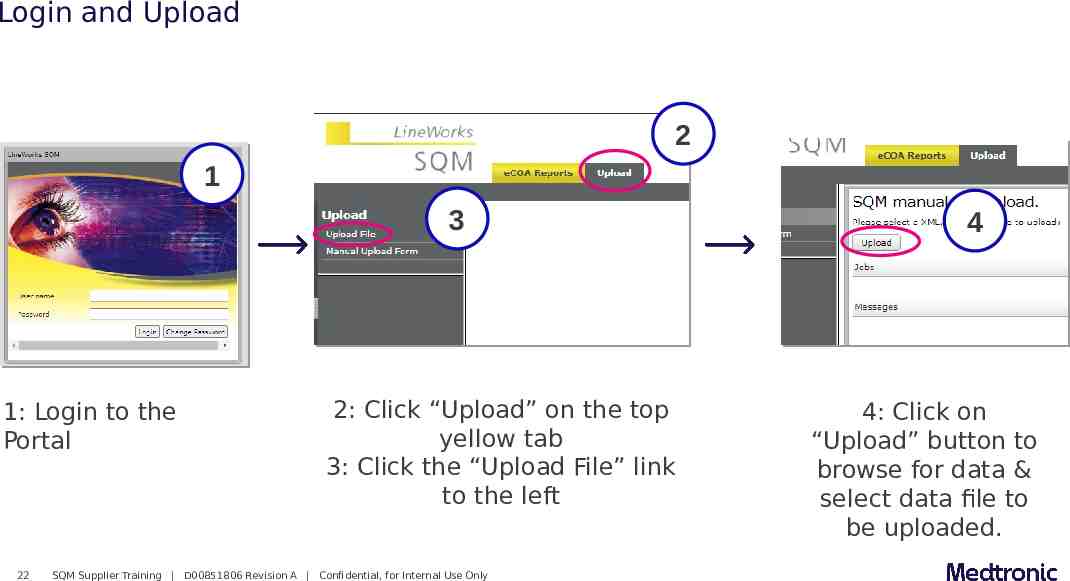
Login and Upload 2 1 3 1: Login to the Portal 22 2: Click “Upload” on the top yellow tab 3: Click the “Upload File” link to the left SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 4 4: Click on “Upload” button to browse for data & select data file to be uploaded.

Data Validation Once the file is uploaded to SQM, a message will appear stating that the data was processed into SQM. A popup window will appear (depending on browser popup settings) with the results of the lot - Pass or Fail (see next slides for example) Pass: all features (variables and attribute data) met specification limits, or other acceptance limits as defined in the SPC form Fail: one or more features were rejected because they did not meet system requirements. 23 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
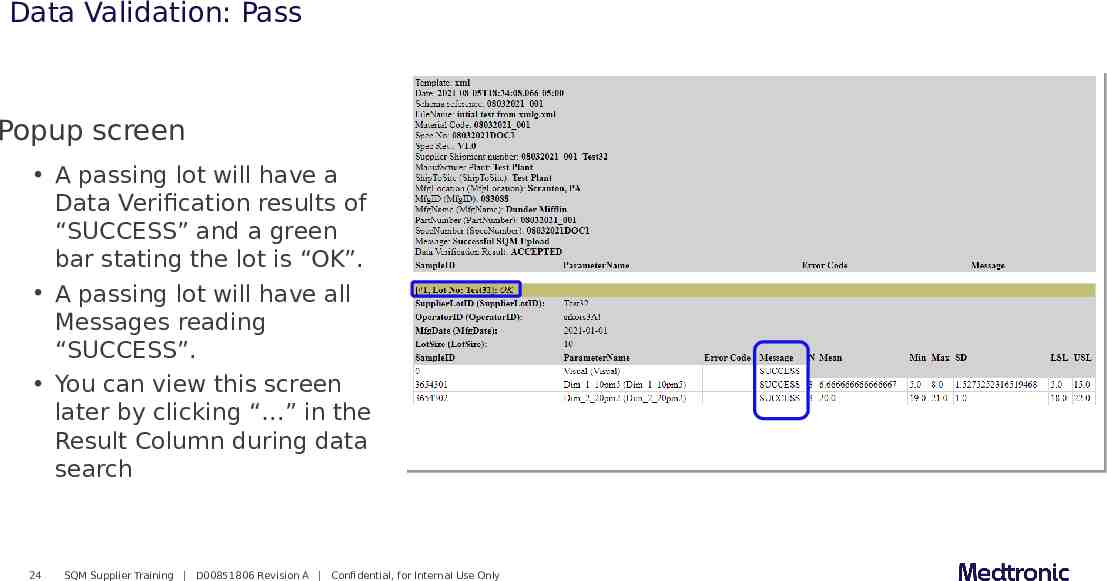
Data Validation: Pass Popup screen A passing lot will have a Data Verification results of “SUCCESS” and a green bar stating the lot is “OK”. A passing lot will have all Messages reading “SUCCESS”. You can view this screen later by clicking “ ” in the Result Column during data search 24 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
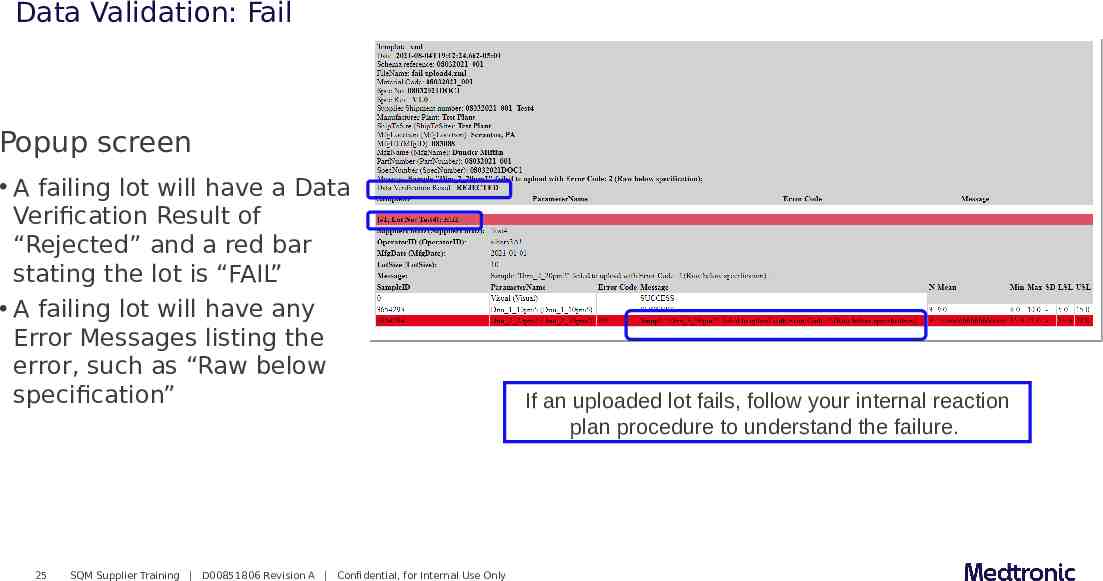
Data Validation: Fail Popup screen A failing lot will have a Data Verification Result of “Rejected” and a red bar stating the lot is “FAIL” A failing lot will have any Error Messages listing the error, such as “Raw below specification” 25 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only If an uploaded lot fails, follow your internal reaction plan procedure to understand the failure.
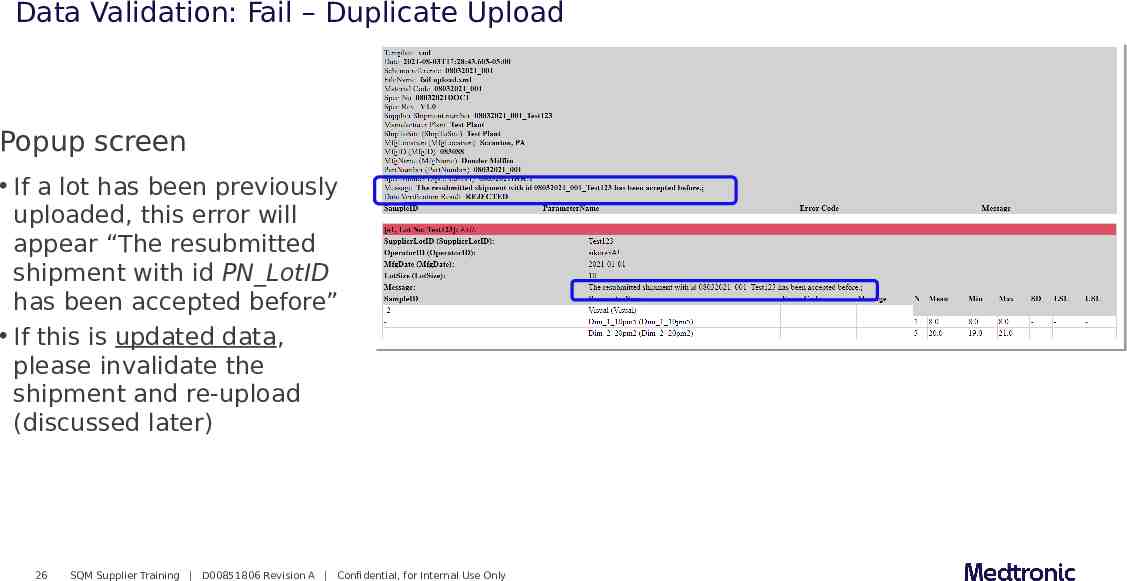
Data Validation: Fail – Duplicate Upload Popup screen If a lot has been previously uploaded, this error will appear “The resubmitted shipment with id PN LotID has been accepted before” If this is updated data, please invalidate the shipment and re-upload (discussed later) 26 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
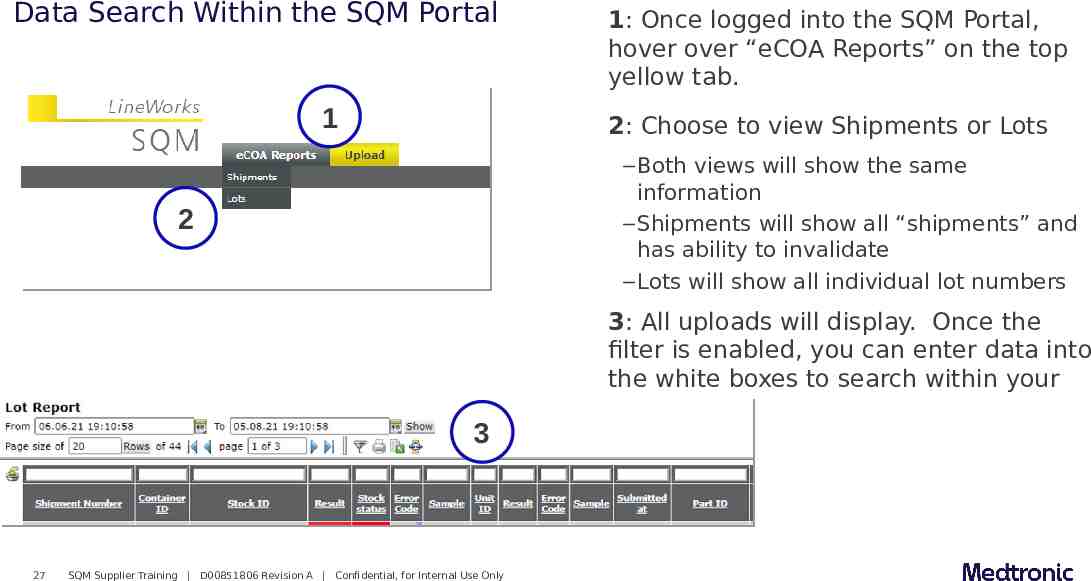
Data Search Within the SQM Portal 1 2: Choose to view Shipments or Lots – Both views will show the same information – Shipments will show all “shipments” and has ability to invalidate – Lots will show all individual lot numbers 2 3 27 1: Once logged into the SQM Portal, hover over “eCOA Reports” on the top yellow tab. SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 3: All uploads will display. Once the filter is enabled, you can enter data into the white boxes to search within your uploads.
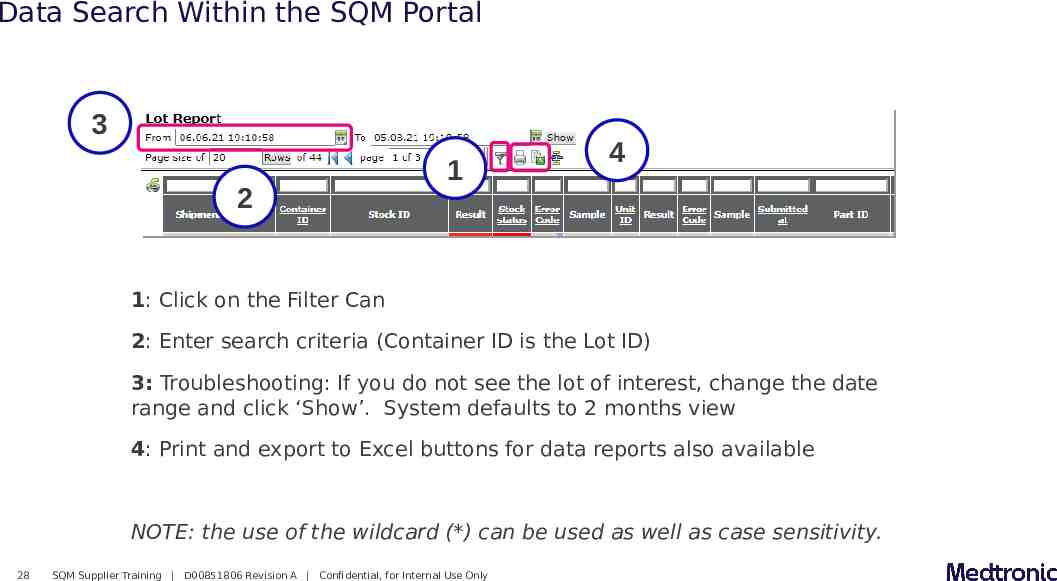
Data Search Within the SQM Portal 3 2 1 4 1: Click on the Filter Can 2: Enter search criteria (Container ID is the Lot ID) 3: Troubleshooting: If you do not see the lot of interest, change the date range and click ‘Show’. System defaults to 2 months view 4: Print and export to Excel buttons for data reports also available NOTE: the use of the wildcard (*) can be used as well as case sensitivity. 28 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
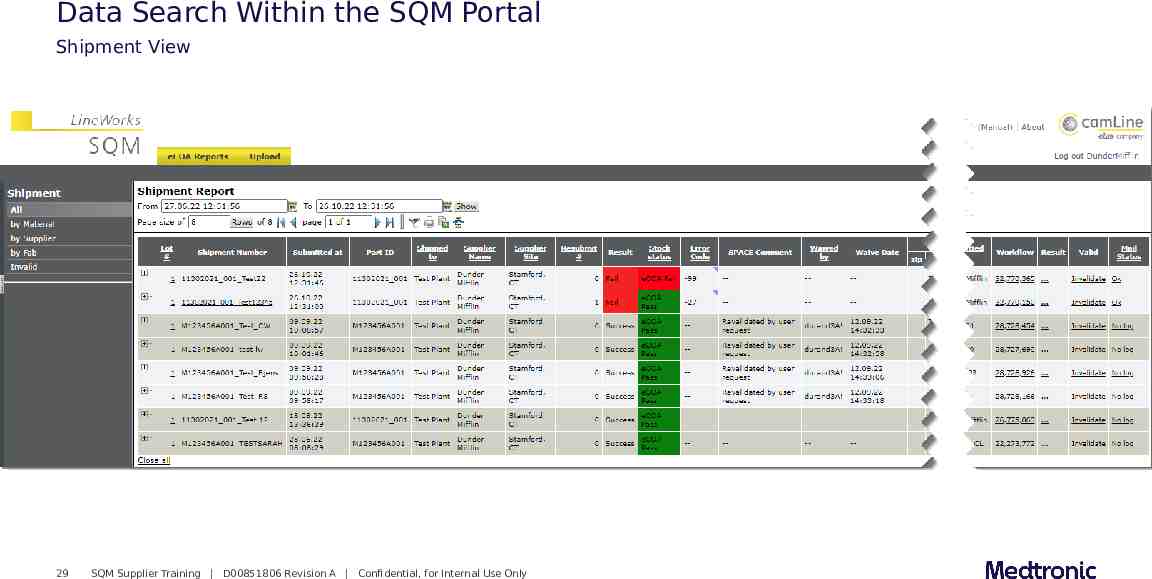
Data Search Within the SQM Portal Shipment View 29 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
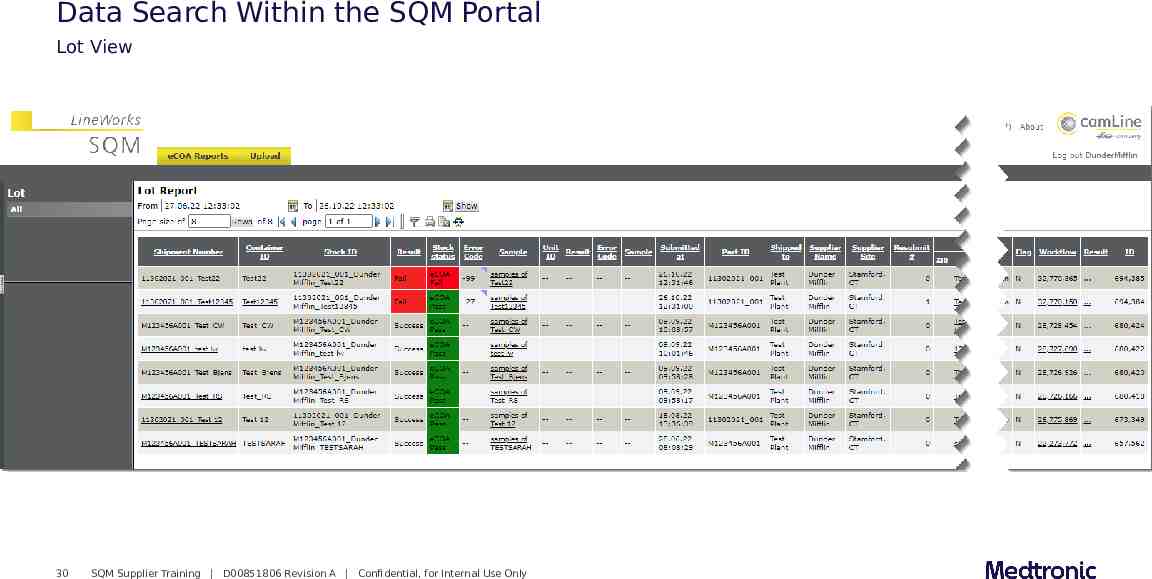
Data Search Within the SQM Portal Lot View 30 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
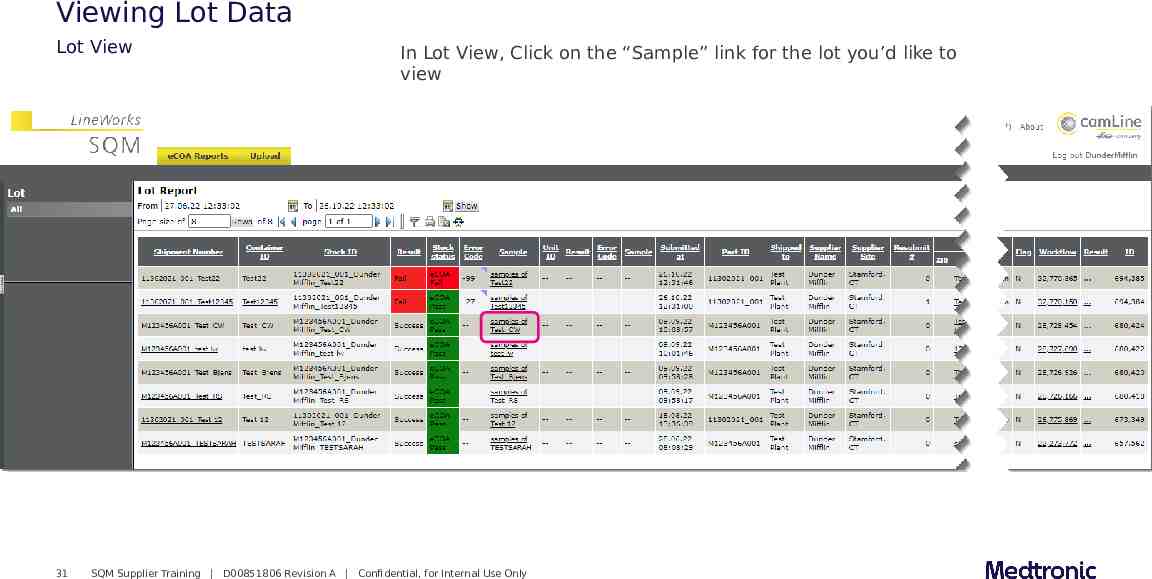
Viewing Lot Data Lot View 31 In Lot View, Click on the “Sample” link for the lot you’d like to view SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
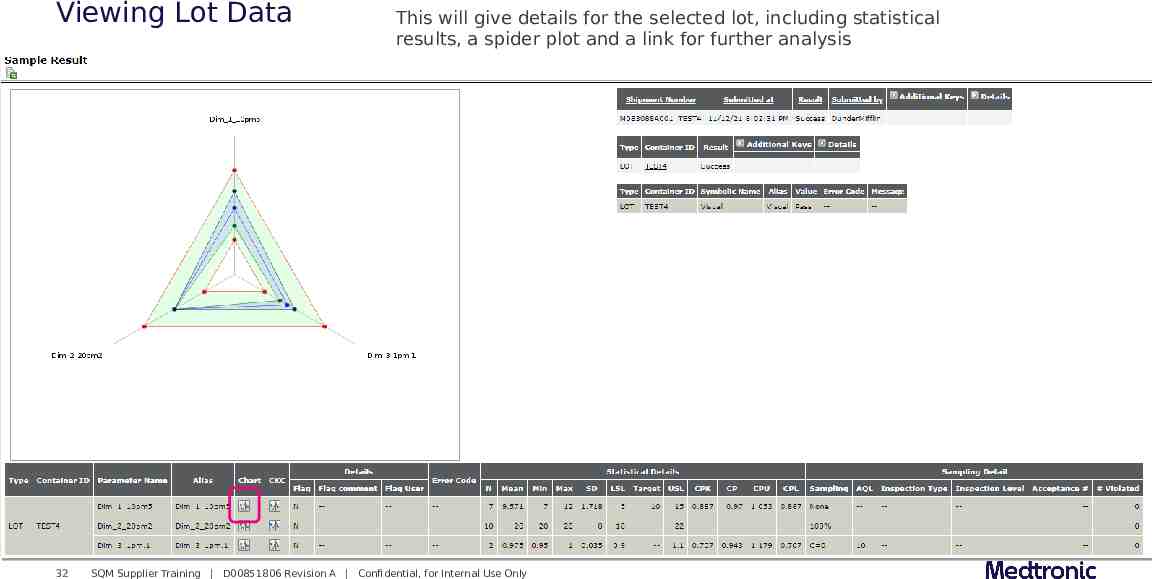
Viewing Lot Data 32 This will give details for the selected lot, including statistical results, a spider plot and a link for further analysis SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
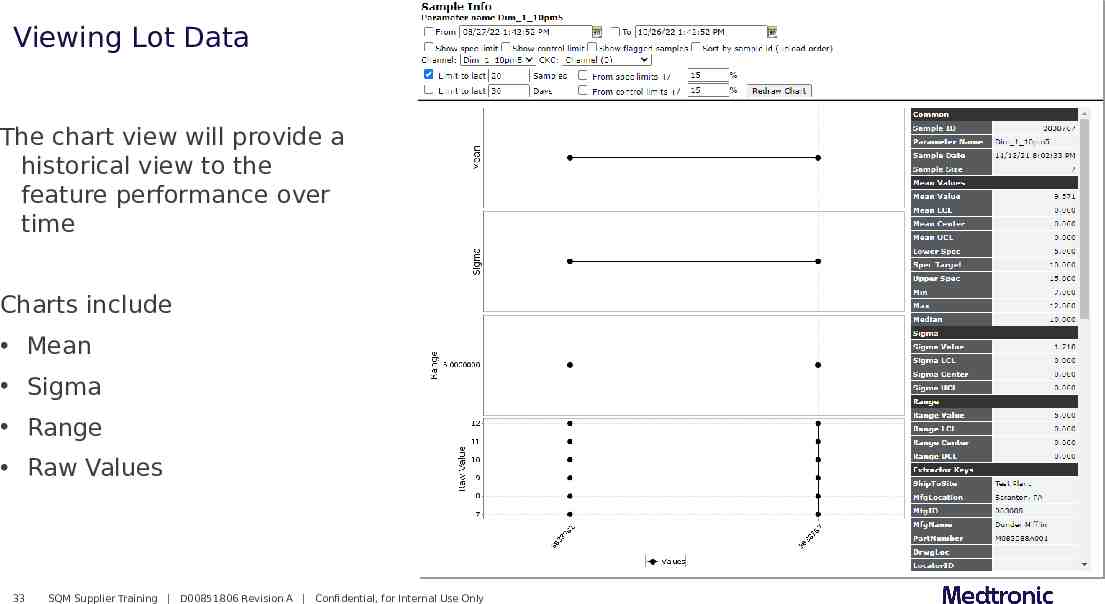
Viewing Lot Data The chart view will provide a historical view to the feature performance over time Charts include Mean Sigma Range Raw Values 33 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Invalidate – Why? Invalidating uploads in the Portal is required when the data cannot/should not be used for product acceptance Duplicate uploads will automatically fail subsequent uploads Examples of when you may need to Invalidate an upload All test uploads. Corrections to incorrect information entered into the XML file. Incorrect Excel Template was used to create the XML file. 34 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
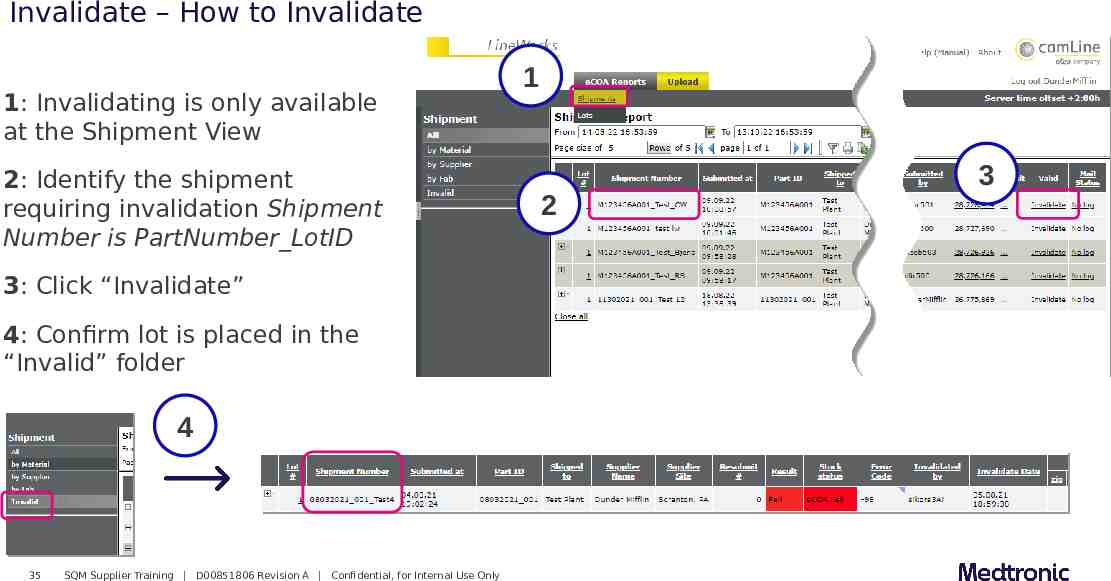
Invalidate – How to Invalidate 1: Invalidating is only available at the Shipment View 2: Identify the shipment requiring invalidation Shipment Number is PartNumber LotID 3: Click “Invalidate” 4: Confirm lot is placed in the “Invalid” folder 4 35 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 1 2 3

Unique Situations
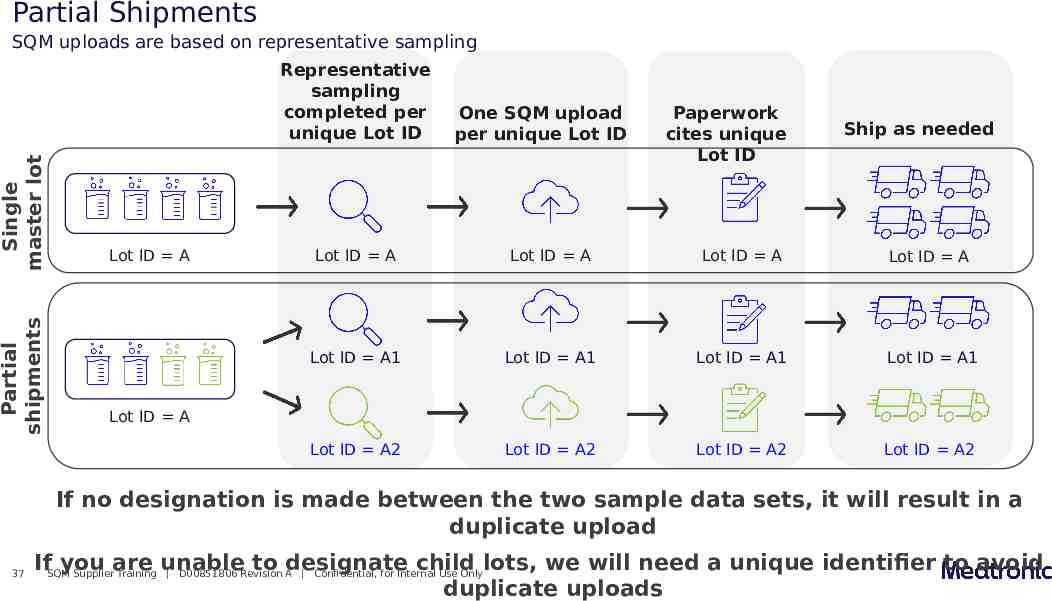
Partial Shipments SQM uploads are based on representative sampling Partial shipments Single master lot Representative sampling completed per unique Lot ID Lot ID A One SQM upload per unique Lot ID Paperwork cites unique Lot ID Ship as needed Lot ID A Lot ID A Lot ID A Lot ID A Lot ID A1 Lot ID A1 Lot ID A1 Lot ID A1 Lot ID A2 Lot ID A2 Lot ID A2 Lot ID A2 Lot ID A If no designation is made between the two sample data sets, it will result in a duplicate upload 37 IfSQMyou are unable to designate child lots, we will need a unique identifier to avoid Supplier Training D00851806 Revision A Confidential, for Internal Use Only duplicate uploads

Sending & Receiving Files for Upload Sending SQM Templates Options for Receiving Files Medtronic will send Macro Enabled Excel Templates for upload. Some companies prevent macros from being received via email. If this occurs, we will work together to transfer the files. Zip file Secure Mail Microsoft 365 Shared Folder Sharepoint Site Microsoft sometimes automatically blocks all macros. Work with your IT department to allow macros from Medtronic. 38 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Troubleshootin g

Common Errors & Issues Error Reason Fix a) The Mfg Date is in the incorrect format a) Verify Excel file or XML file. Mfg date should be YYYY-MMDD format b) The Lot size is the incorrect format c) Used wrong Excel template to generate upload file d) Your account doesn’t have correct permissions b) The lot size cannot have comma’s. Remove comma (Ex 1,000 vs 1000) c) Verify Excel template is latest version. Contact help desk ( [email protected] m ) for verification d) Contact the help desk ( [email protected] m ) for resolution. Include a screenshot or ID number 40 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Common Errors & Issues Error Reason Fix 1) Re-save the PDF with a slightly different name and try uploading 41 Trying to upload a zip file and no response after clicking “Upload” A bug in the system prevents certain part/lot number combinations from being uploaded – we are working on a fix SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 2) Send zip file to [email protected] om with the word “Medtronic ZIP File” in the subject line to ensure delivery. The help team will upload the zip file on your behalf and send a note when it is uploaded successfully

Common Errors & Issues Error Reason Fix 42 “No permission to change password” or “Error occurred in Lineworks” when trying to change password New password doesn’t meet Medtronic Password Policy SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only Change password according to Medtronic Password Policy: Password must be between 710 characters Not allowed to use any of your past 10 passwords. Uppercase/Lowercase characters (or combination). Numeric (0-9) characters Password cannot contain a user’s account name. It is strongly advised that passwords should not contain dictionary words.

Common Errors & Issues Error Reason Fix Enable pop-up windows from sqm.medtronic.com Click on “Show Result” Navigate to Shipment or Lot view and click “ ” under Result column for specific Lot number 43 No Result screen pop-up appears Browser has pop-up windows disabled SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Resources
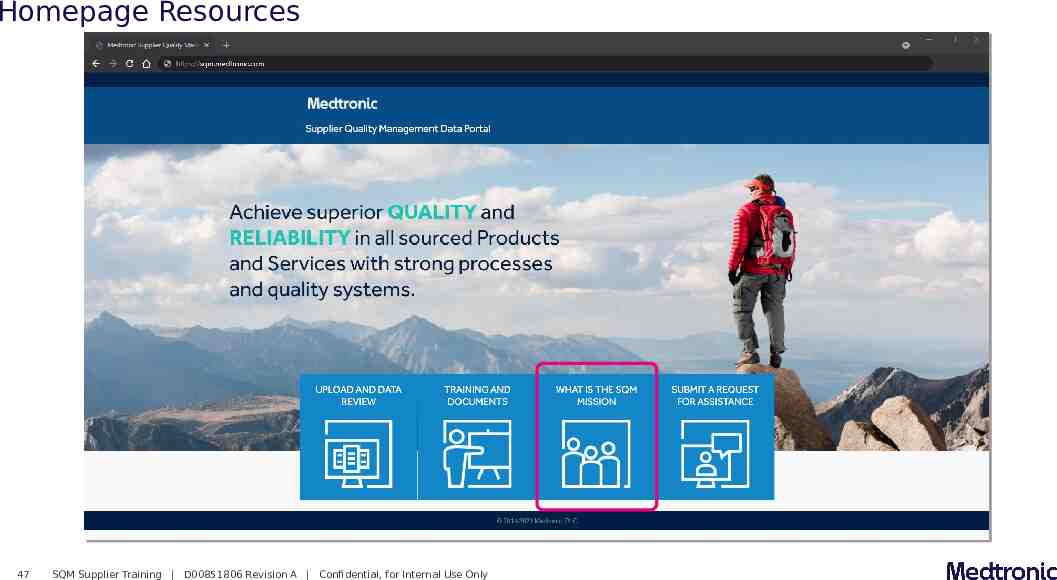
Homepage Resources 45 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
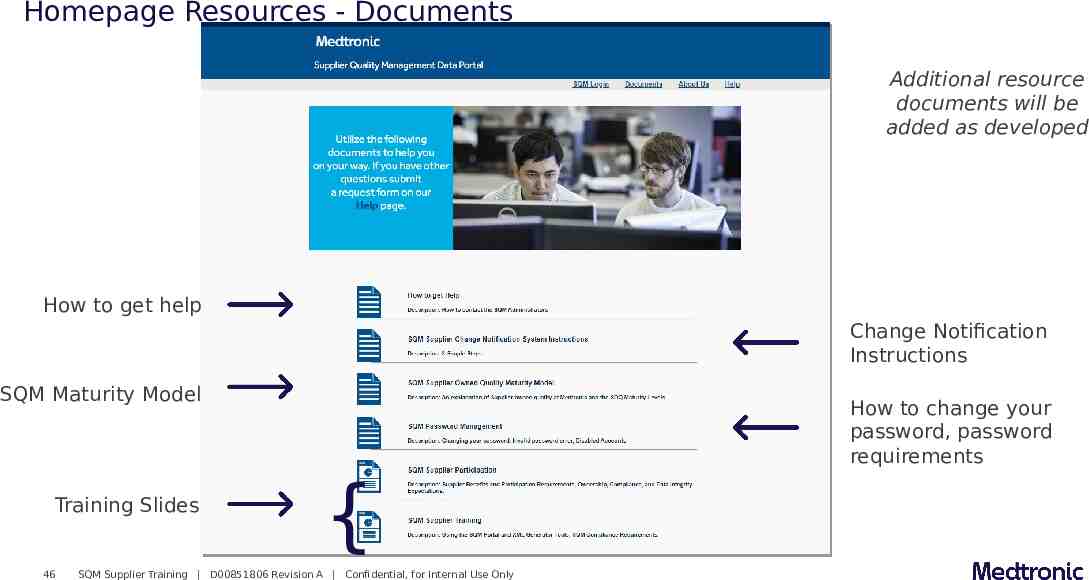
Homepage Resources - Documents Additional resource documents will be added as developed How to get help Change Notification Instructions SQM Maturity Model Training Slides 46 How to change your password, password requirements { SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
Homepage Resources 47 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
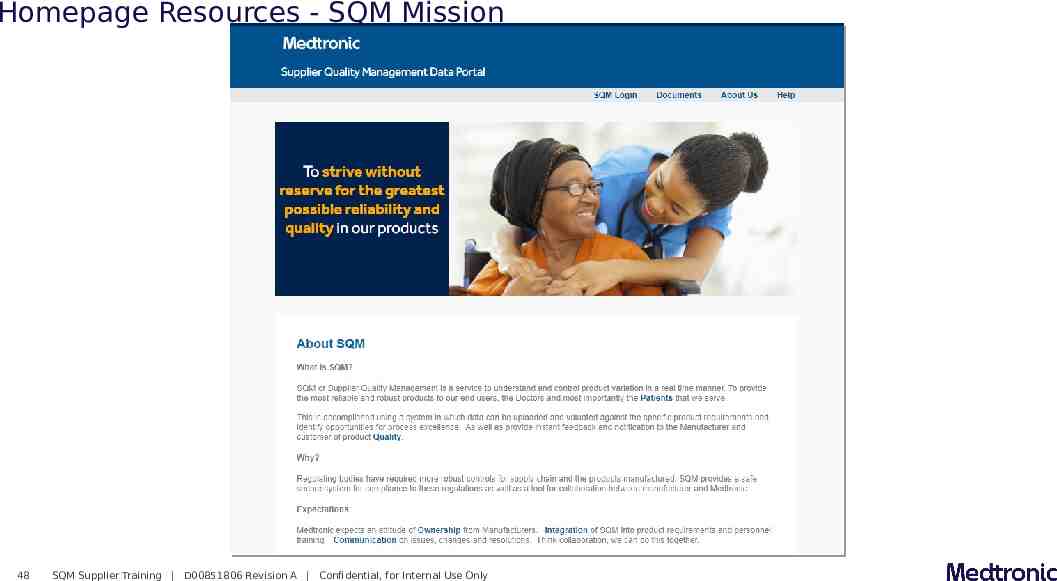
Homepage Resources - SQM Mission 48 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
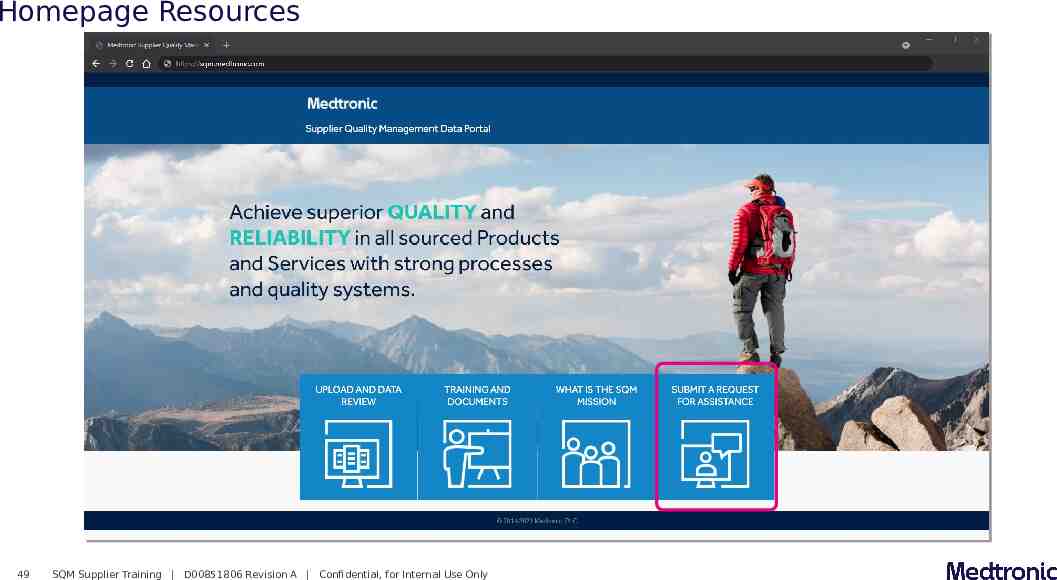
Homepage Resources 49 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only
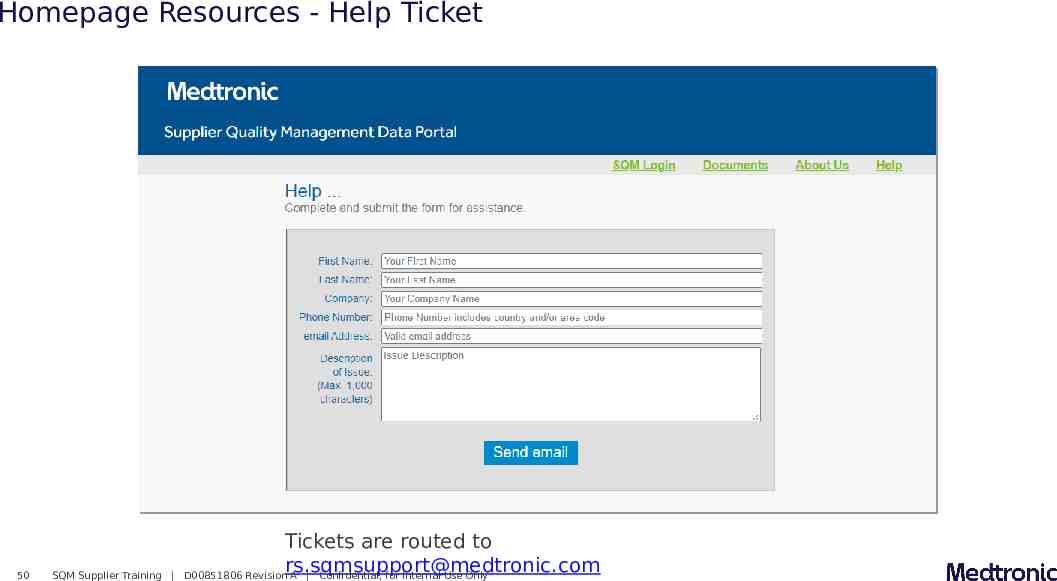
Homepage Resources - Help Ticket 50 SQM Supplier Training Tickets are routed to [email protected] D00851806 Revision A Confidential, for Internal Use Only

Supplier Change Notification Reminder We recognize that continuous improvement efforts at suppliers are always occurring. With that, collaboration and communication are key to ensure changes don’t have unintended effects on our products. As part of our partnership, suppliers must notify Medtronic of changes being considered to materials, products, or processes. Medtronic needs your help with these items when a change is being initiated: If not already done establish an effective process for triggering notifications to Medtronic (SCN) Please provide as much advance notice as possible Identify in the change if the part(s) is on SQM for us to evaluate if changes to setup need to occur Please work with your Medtronic representative to help you through the change notification process 51 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only SCR Portal Link: https://wwwp.medtronic.com/changerequest/public/landingPage

[email protected] om For all other issues or questions, please do not hesitate to reach out to the help desk via website ticket or direct email. This will ensure fastest response time and resolution. 52 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Next Steps
Next Steps SQM Process implementation – utilize Medtronic Work Instruction or create your own Sign SPC forms Receive SQM Excel Templates Go Live! QUESTIONS ? 54 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only

Than k you!

Backup Slides
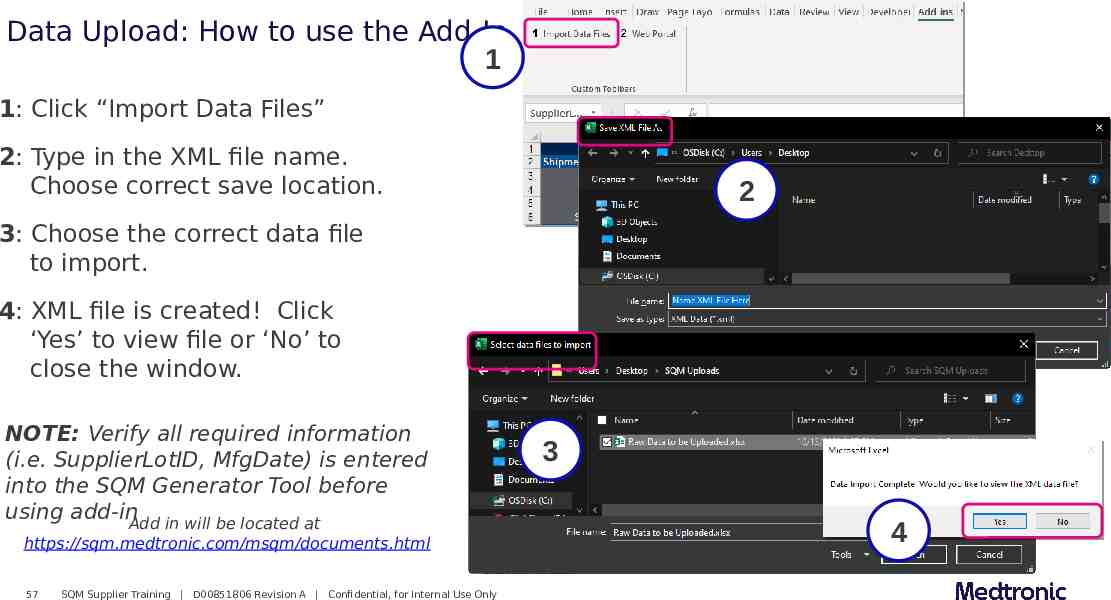
Data Upload: How to use the Add-In 1 1: Click “Import Data Files” 2: Type in the XML file name. Choose correct save location. 2 3: Choose the correct data file to import. 4: XML file is created! Click ‘Yes’ to view file or ‘No’ to close the window. NOTE: Verify all required information (i.e. SupplierLotID, MfgDate) is entered into the SQM Generator Tool before using add-inAdd in will be located at https://sqm.medtronic.com/msqm/documents.html 57 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only 3 4
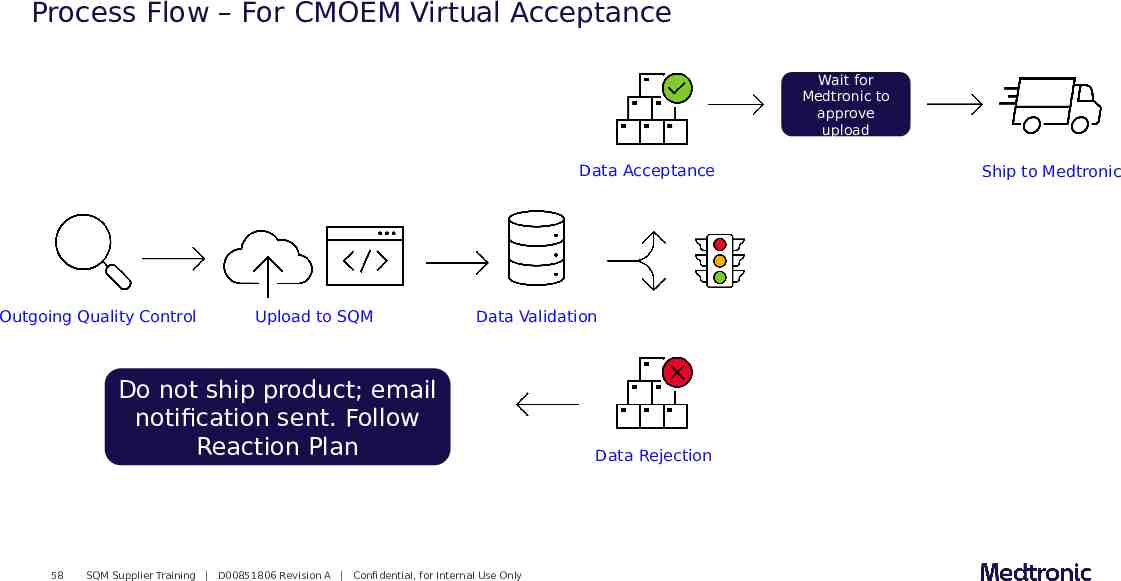
Process Flow – For CMOEM Virtual Acceptance Wait for Medtronic to approve upload Data Acceptance Outgoing Quality Control Upload to SQM Data Validation Do not ship product; email notification sent. Follow Reaction Plan 58 SQM Supplier Training D00851806 Revision A Confidential, for Internal Use Only Data Rejection Ship to Medtronic






