Respecifying Measures to Electronic Clinical Quality Measures (eCQMs)
18 Slides3.72 MB

Respecifying Measures to Electronic Clinical Quality Measures (eCQMs) August 2020 Presenter: -Brenna Rabel (Battelle)

Vision and Goals An ongoing process to engage the public in quality measure development. Elicit feedback that will help CMS design resources that can help all of those interested in healthcare quality improvement better understand the goals of quality measurement. 2 Education Outreach Dedicated Websites Measure Development Roadmaps Listserv opportunities 2/12/2020

Overview Define Measure Respecification Discuss Rationale for Respecification Determine Measure Suitability for Respecification – Understand Feasibility Testing Considerations Review Requirements for eCQM Specifications Identify Appropriate eCQM Data Elements Development and Use of a Checklist 3

What is Respecifying1? An existing measure is changed to fit the current purpose or use. This may mean changing a measure to meet the needs of a different care setting, data source, or population. Or, it may mean changes to the numerator, denominator, or adding specifications to fit the current use. For example, transition existing paper-based, claims, or registry measure to an eCQM. 4 1 Source: CMS Blueprint for the CMS Measures Management System

Why Respecify to an eCQM? 5 Program requirement for EHR data and eCQM specification Reduce burden of manual abstraction Use measure in a different setting Use measure in a different population Have a different attribution level

To Respecify or Not To Respecify to an eCQM Does it meet the importance criterion? – Is the proposed eCQM applicable to the new setting or population? – Does it meet a quality goal of the new setting or population? Does it meet the feasibility criterion? – – – – – – 6 Are there any anticipated attribution-related challenges? What is the EHR adoption rate for the proposed care setting? Is there any undue burden or significant clinical workflow impact? Can all data elements be mapped to EHR data? Can the measure be re-specified using current standards ? Can the measure be reported to CMS using current standard s?

Determine if the Measure can be Respecified 7
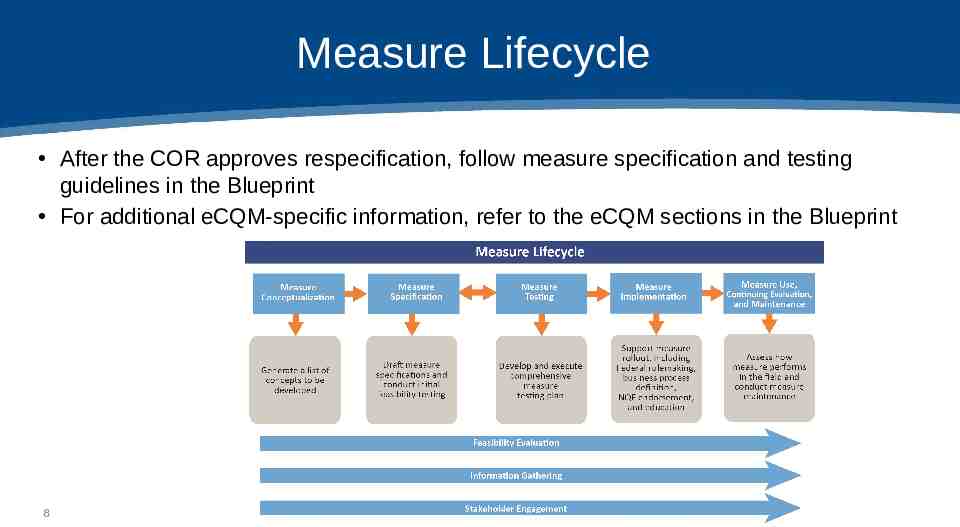
Measure Lifecycle After the COR approves respecification, follow measure specification and testing guidelines in the Blueprint For additional eCQM-specific information, refer to the eCQM sections in the Blueprint 8

Considerations when Respecifying to an eCQM Data elements from one data source e.g., claims, do not always translate 1:1 to an eCQM – Recommend using the NQF Feasibility Scorecard as early as possible – Do not make assumptions that all required data elements for the measure are collected and stored in an EHR in a structured data field 9

eCQM Standards To address disparate EHRs, health information technology (IT) standards and tools are used in authoring eCQMs The standards are managed by the health IT community via a consensusbased development process and adopted for use in CMS programs Current eCQM standards – Quality Data Model (QDM), Clinical Quality Language (CQL), Health Quality Measure Format (HQMF), and Quality Reporting Document Architecture (QRDA) Current tools – Measure Authoring Tool (MAT), Bonnie, CQL-to-Expression Logical Model (ELM) Translator, Value Set Authority Center (VSAC), Cypress 10

Review of eCQM Standards 11 2 Source: https://ecqi.healthit.gov/qrda

Relationship of eCQM Tools with Standards 12

eCQM Feasibility Testing Feasibility Testing must be done to assess feasibility and determine the extent to which the required data are available and retrievable without undue burden, and the extent to which they can be implemented for performance measurement.4 Test early and often; engage stakeholders early and often Fail early to inform respecification Do not expect the same performance results of eCQM testing as with the original measure 13 4 Source: CMS Blueprint for the CMS Measures Management System

Determine Data Elements 14

eCQM Feasibility Testing: Points to Consider Will there be workflow or technology changes required for the eCQM? Will testing the eCQM show it will be limited to an integrated healthcare delivery system with a shared IT system or interoperable EHR system? Will the eCQM require data from another setting e.g., facilitybased data for an eligible clinician eCQM? 15

Additional References For eCQM Standards: – Electronic Clinical Quality Improvement (eCQI) Resource Center (QDM) – HL7 Standards (CQL, HQMF, QRDA) For eCQM Tools – – – – – MAT National Library of Medicine VSAC Bonnie CQL-to-ELM Translator Cypress For eCQM Testing: – Introduction to eCQM Testing – National Quality Forum Feasibility Scorecard 16

Upcoming Webinars Understanding Clinical Quality Measures: How CMS is modernizing its approach to digital measurement Presented by Kim Rawlings (CMS), Nicole Brennan (Battelle), and Brenna Rabel (Battelle) – September 15th, 2-3pm ET – September 17th, 3-4pm ET 17

Questions 18






