Protecting Human Participants in Research research
37 Slides128.76 KB

Protecting Human Participants in Research http://research integrity.syr.edu/ Office of Research Integrity and Protections (ORIP)

What Is the Institutional Review Board and what is it’s role?

Composition of the IRB The IRB must be comprised of at least 5 members from relevant and diverse academic disciplines and include at least one non-affiliated community member.

Role of the IRB The IRB is designated to protect human subjects participating in research conducted at or sponsored by an institution. The IRB is responsible for the review and approval of ALL research projects involving human subjects for compliance with institutional, state, local and federal laws and/or regulations as well as the ethical principals contained in the Belmont Report.

The Belmont Report Basic Ethical Principles 1. 2. 3. Respect for Persons Beneficence-(1) do not harm and (2) maximize possible benefits and minimize possible harms. Justice- fair procedures and outcomes in the selection of research subjects

Federal Policy for the Protection of Human Subjects Common Rule 45 CFR 46 (Part A) Regulations for: The roles and responsibilities of the IRB The definition of research Categories for review of research Criteria for IRB review/approval of research General requirements for informed consent

Definition of Human Subjects Research as defined in 45 CFR 46.102(e) Research means a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Systematic investigation-attempts to answer a research question; is methodically driven-collects data in an organized and consistent way; attempts to analyze data or information in some manner; and intends to draw conclusions from the result. Generalizable knowledge-contributes to a theoretical framework of an established body of knowledge; publication; presentation; workshop; conference; results are intended to inform the field of study; web based publications, etc.
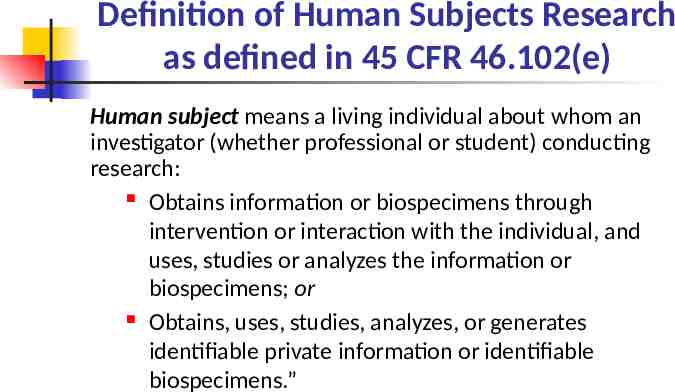
Definition of Human Subjects Research as defined in 45 CFR 46.102(e) Human subject means a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies or analyzes the information or biospecimens; or Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”

Definition of Human Subjects Research as defined in 45 CFR 46.102(e) Intervention includes both physical procedures by which information or biospecimens are gathered and manipulations of the subject or the subject's environment that are performed for research purposes. Interaction includes any method of communication or interpersonal contact between investigator and subject. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information that has been provided for specific purposes by an individual and that the individual can reasonably expect will not be made public. Identifiable private information/biospecimen is private information for which the identity of the subject/biospeicmen is or may be readily ascertained by the investigator or associated with the information/biospecimen.
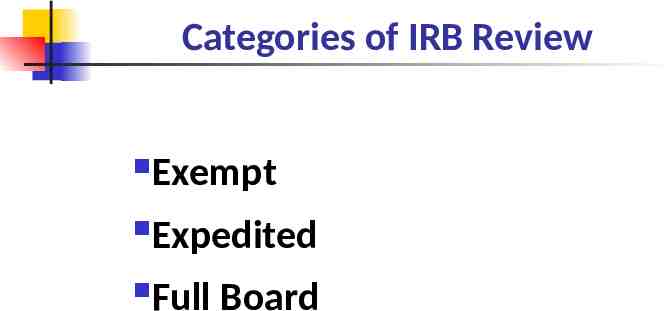
Categories of IRB Review Exempt Expedited Full Board

The category of research is determined by the level of risk to the participant.

Exempt Review Exempt research studies present risks so benign, that the federal regulations determine these types of studies to be exempt from review. Studies that meet the definition of human subjects research may qualify for exemption under one or more of the 8 categories defined by the Federal Regulations. At SU, exemption must be determined by the IRB, not the investigator.

Exempt Review Most common examples of Exempt Research Activities: Anonymous surveys Research on regular/special educational instructional strategies that do not interfere with students’ ability to learn required content or adversely affect the assessment of those providing instruction. Research involving benign behavioral interventions that are brief in duration, harmless, painless, not physically invasive, are not likely to have a significant adverse lasting impact, and/or will not be offensive or embarrassing (playing an online game, solving puzzles under various conditions, etc.). Research involving deception ONLY if the subject agrees to participate in research circumstances in which they are informed that they will be misled regarding the purpose of the research.

Exempt Review Process Exempt applications are reviewed by the ORIP Director, Tracy Cromp. Turn around time for review is approximately 5-7 business days. There are no deadlines for exempt applications. Allow a minimum of 4 weeks for the review process. Exempt studies are authorized for a period of 5 years.

Expedited Review Expedited research activities: (1) present no more than minimal risk to human subjects, and (2) involve only procedures listed in one or more of the 9 categories defined by the Federal Regulations.
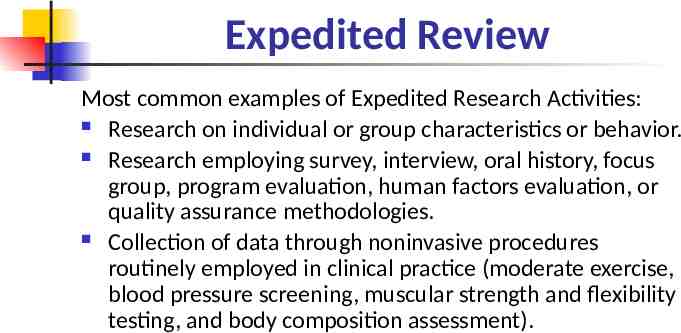
Expedited Review Most common examples of Expedited Research Activities: Research on individual or group characteristics or behavior. Research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. Collection of data through noninvasive procedures routinely employed in clinical practice (moderate exercise, blood pressure screening, muscular strength and flexibility testing, and body composition assessment).

Expedited Review Process Expedited applications are reviewed by the IRB Chair or one of the IRB Co-Chairs and, when deemed necessary, specialists in the field being studied. Turn around time for review is approximately 7-10 business days. There are no deadlines for expedited applications. Allow a minimum of 4 weeks for the review process. Expedited studies are approved until the project is closed. Continuing renewal is no longer required; however, an Annual Research Status Report will be requested.

Full Board Review Full Board research activities involve greater than minimal risks to participants.

Full Board Review The research involves greater than minimal risk if: Involvement in the research exposes the participants to harm or discomfort beyond level encountered in daily life. Disclosure of the participants’ responses outside of the research could place them at risk of criminal or civil liability, be damaging to their social or financial standing, employability, or reputation

Full Board Review Full Board Research Activities may involve: Potential illegal behavior Under age drinking Situations of abuse, harm, or neglect Sensitive topics that could be damaging to participants Disclosure of medical status Sexual behavior Job satisfaction Legally restricted participants

Full Board Review Process Full board applications must be reviewed at a convened meeting of the IRB with a majority of IRB members in attendance. There is a deadline for full board applications. Full board applications must be received two weeks prior to the scheduled IRB meeting. This is hard deadline. The IRB meets monthly except during the month of July. The meeting schedule is posted on our website.

Full Board Review Process Allow a minimum of 8 weeks for the approval process. Full board applications can be approved for up to 365 days from the date of review (unless the IRB determines more frequent review is necessary). Annual continuing review at a convened meeting of the full board is required. Full board studies are renewable for up to 7 years.

Application Content Purpose of Research (Rationale or research question) Provide a lay description of the proposed research and the hypothesis to be evaluated. Approach/Method –Describe the methods that will be used to gather the data. Define what you will ask participants to do. The qualifications of the Researchers listed in the protocol. The characteristics of the participants-including any special populations. Any possible participant risks-physical, psychological, financial, etc. and the procedures that will be used to mitigate the risks. Any possible participant benefits and how those benefits outweigh the risks. How participant privacy and confidentiality of collected data will be maintained. How participants will be recruited (learn about participation in the research). The type of informed consent that will be obtained.

Application Information Student researchers cannot be Principal Investigators on Human Subjects research projects that require IRB review and oversight at SU. It is the policy of the SU IRB that the Principal Investigator be a member of the SU faculty at one of the following positions/levels: Assistant, Associate, Full Professor; Academic, Research, or Professor-of Practice, Department Dean/Chair; or Administrative Staff with the position of Director of higher. All applications should be completed under the guidance of the student’s faculty advisor and reviewed by the faculty advisor prior to submission. .

Informed Consent/Assent Consent is required for all human subject participants 18 years of age or older. Assent is required for all human subject participants who are minors (17 years of age or younger) or those considered impaired in their decision making ability. Although you may request a waiver of the documentation of written consent/assent, i.e. electronic or oral consent/assent, you must obtain consent/assent from all participants who will engage in your study.

Informed Consent Informed consent is a voluntary agreement to participate in research. It is not simply a document but it is also a process. The goal of the informed consent process is to provide sufficient information so that a reasonable person can make an informed decision about whether to enroll in a study and/or to continue participation. Informed Consent must be obtained from all adults aged 18 or older or the legally authorized representative prior to any engagement in human subjects research.

Informed Consent Obtaining consent involves informing prospective participants or the legally authorized representative about their rights and providing them with key information regarding involvement in the research and documenting the process. The consent process should be a dialogue that includes a clear description of the study’s purpose, duration, procedures, alternatives, potential risks, and benefits. Circumstances should allow the prospective participant or the legally authorized representative the opportunity to ask questions and provide sufficient time to consider whether or not to participate. Because consent is an ongoing process, it must be clear that participation is voluntary and that participants have the right to withdraw from the study at any time, not just at the time of initial consent.

Informed Consent General requirements for Written, Oral and/or Electronic Consent: Must begin with a concise and focused description of the purpose for the research (using language at a reading/comprehension level of the targeted population. Information regarding the procedures. Descriptions of all research activities, including their purpose and duration. Descriptions of the types of measures you will use, including an explanation as to who will administer them. A description of any possible risks and/or discomforts associated with participation and how the risks will be mitigated. A description of any possible benefits associated with participation. A description of how the privacy interests of the participant will be protected A description of how the confidentiality of the data will be maintained. A description of participant rights including a statement that participation is voluntary. An explanation of who to contact for answers to pertinent questions.

Informed Consent General requirements for Written, Oral and/or Electronic Consent: Any research activities that involve the collection of identifiable private information or identifiable biospecimens must include one of the following: A statement that identifiers might be removed from the identifiable private information /biospecimens and after such removal, the information/biospecimens could be used for future research studies or distributed to another investigator for future research studies without additional informed consent from the subject or the legally authorized representative, if this might be a possibility; or A statement that the subject's information/biospecimens collected as part of the research, even if identifiers are removed, will not be used or distributed for future research studies.

Informed Consent Additional Requirements for Written, Oral and/or Electronic Consent when applicable: A description of any alternatives to participation. A description of any medium of recording (photographs, audio, video, film) which includes the purpose for the recording, how they will be used, who will have access to them, and the disposition of them when the study is complete . A description of whether compensation will be offered which includes the method of compensation, how it will be awarded, and how it will be pro-rated if a participant withdraws prior to completion. Information regarding situations of abuse, abuse or harm and a description of if/when mandated reporting is indicated. Information regarding legal subpoena. Information regarding Certificates of Confidentiality. Information about whether relevant research results will be returned to the participants. Information about possible commercial profit. Information about whether research activities will include whole genome sequencing.

Completed IRB Application In order to prevent additional delays in the review process a complete IRB application must include: The finalized versions of all research instrumentsquestionnaires/surveys/interview guide questions/focus group topics, etc. All recruitment tools, scripts for direct-face-to-face/telephone/announcements, Internet/social media posts, advertisements, flyers, emails, letters, etc. All informed consent/assent documents as appropriate. Any additional forms that may be required, letters of cooperation, international research, research conducted in schools, children, prisoners, genetic research, etc.

Outcomes of Review Once your application has been submitted, the IRB can: Approve Request modification(s) or additional information Disapprove Re-Categorize the research as Exempt, Expedited or Full

Student Projects Student projects involving human participants that are conducted solely to fulfill course requirements do not require IRB review. However, if data will be shared beyond the classroom/course assignment (e.g. in any type of publication-including web publication, for presentation at academic conferences/workshops, in thesis/dissertation, etc.) the project must receive IRB approval prior to initiation. All Capstone, Honor’s, Master’s or doctoral theses involving human participants must be submitted for IRB review.

Training Requirements CITI Training is required for all Expedited and Full Board Applications. All persons listed in the protocol application that will have direct contact with participants and/or identifiable human participant data are required to complete the CITI training appropriate to their role in the research. No Expedited or Full Board studies will be approved until CITI training requirements are satisfied.

Amendments Amendments are required whenever any are made to the materials in the original approved protocol. Changes to the protocol, no matter how minor, cannot be implemented prior to IRB review and formal approval of the changes. - Exception: if the change is essential to protect human participants from harm

Reminders . No human subjects research can be conducted without No human subjects research can be conducted without IRB approval or exemption. Allow enough time for your application to be approved, it can take up to two months if revisions are needed.

Where do I go for help? The Office of Research Integrity and Protections 214 Lyman Hall Phone Number: 315-443-3013 Email: [email protected] Web: http://researchintegrity.syr.edu






