MATTER Ch. 8 – Solids, Liquids, & Gases III. Behavior of Gases
10 Slides456.00 KB

MATTER Ch. 8 - Solids, Liquids, & Gases III. Behavior of Gases (p.228-231) Pressure Boyle’s Law Charles’ Law
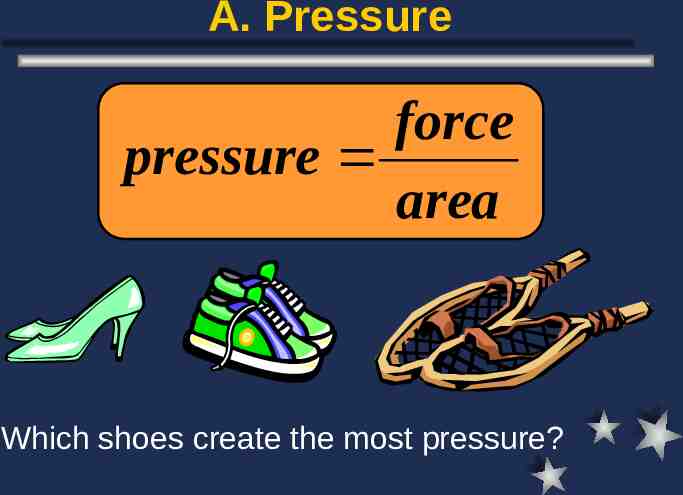
A. Pressure force pressure area Which shoes create the most pressure?

A. Pressure Key Units at Sea Level 101.325 kPa (kilopascal) 1 atm 760 mm Hg 14.7 psi N kPa 2 m

Contained Pressure Atmospheric Pressure A. Pressure Barometer Manometer

A. Pressure Effect on Boiling Point When atmospheric pressure increases, the boiling point of a liquid increases. EX: high altitude cooking, boiling cold water
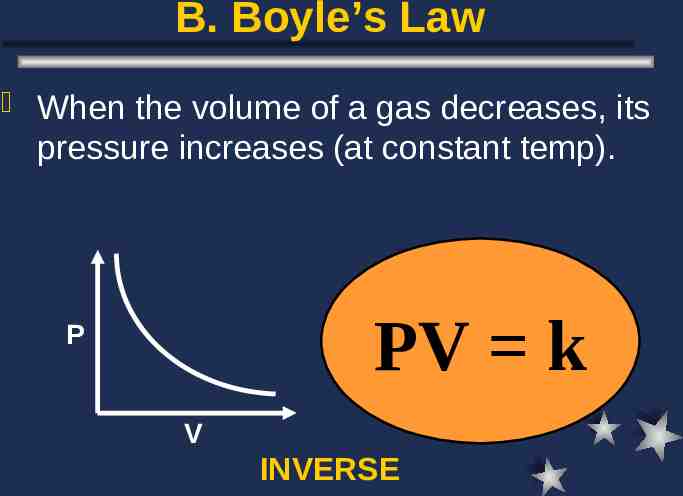
B. Boyle’s Law When the volume of a gas decreases, its pressure increases (at constant temp). PV k P V INVERSE

B. Boyle’s Law

C. Charles’ Law When the temperature of a gas increases, its volume also increases (at constant pressure). V k T V T DIRECT

C. Charles’ Law

C. Charles’ Law Absolute Zero - Temp at which. the volume of a gas would equal zero. all particle motion would stop. -273 C or 0K






