Transgenic Plants (Genetically modified organisms (plants) Genetically
43 Slides7.68 MB

Transgenic Plants (Genetically modified organisms (plants) Genetically engineered plants))

Why create transgenic plants? When there is no naturally occurring genetic variation for the target trait. Examples: 1. Glyphosate herbicide resistance in soybean, corn 2.Vitamin A in rice 3.Blue roses

What genes to transfer? 1. One gene to a few genes - the CP4 ESPS example 2. Multiple genes - Golden Rice and Applause rose 3. In principle, any gene (or genes) ORIGIN 1 61 121 181 241 301 cctttcctac tcactctgga caggaacagc tgtctgcagc cacgccgcgc ctgagtgagg agaggcgtag gcaccagccg aggccaccca gcaaacatct atgctgactc tgaatgggcc cagtcctccg gaacagctcc ggtagaagca gccaaagcct gtctgtccat ggcgggatgc cgggagctgg agttgaccaa cggctccaat ggcggcttgg agttcaaccc tatgaaggag tacatgatct tgagtgatgc gcagcagatc gctgtggcgg tgctgtgtac cctgatgggg ctgctgagtg ccctggagaa cgtggctgtg ctctatctca tcctgtcctc gcagcggctc
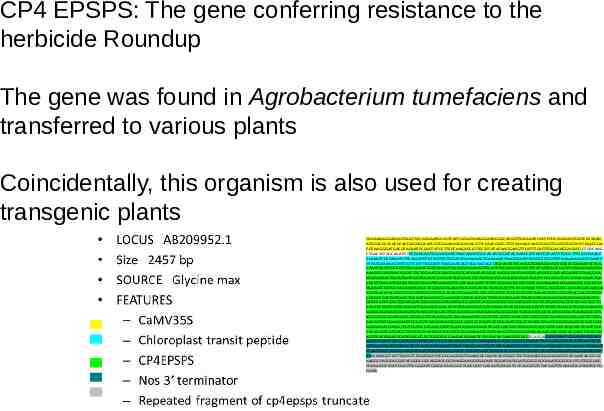
CP4 EPSPS: The gene conferring resistance to the herbicide Roundup The gene was found in Agrobacterium tumefaciens and transferred to various plants Coincidentally, this organism is also used for creating transgenic plants TGGAAAAGGAAGGTGGCTCCTACAAATGCCATCATTGCGATAAAGGAAAGGCCATCGTTGAAGATGCCTCTGCCGACAGTGGTCCCAAAG ATGGACCCCCACCCACGAGGAGCATCGTGGAAAAAGAAGACGTTCCAACCACG TCTTCAAAGCAAGTGGATTGATGTGATATCTCCACTGA CGTAAGGGATGACGCACAATCCCACTATCCTTCGCAAGACCCTTCCTCTATATAAGGAAGTTCATTTCATTTGGAGAGGACACG CTGACAAG CTGACTCTAGCAGATCTTTCAAGAATGGCACAAATTAACAACATGGCACAAGGGATACAAACCCTTAATCCCAATTCCAA TTTCCATAAACC CCAAGTTCCTAAATCTTCAAGTTTTCTTGTTTTTGGATCTAAAAAACTGAAAAATTCAGCAAATTCTATGTTGGTTTTGAAAAAAGATTCAATT TTTATGCAAAAGTTTTGTTCCTTTAGGATTTCAGCATCAGTGGCTACAGCCTGCATGCTTCACGGTGCAAGCAGCCGGCCCGCAACCGCCCG CAAATCCTCTGGCCTTTCCGGAACCGTCCGCATTCCCGGCGACAAGTCGATCTCCCACCGGTCCTTCATGTTCGGCGGTCTCGCGAGCGGTG AAACGCGCATCACCGGCCTTCTGGAAGGCGAGGACGTCATCAATACGGGCAAGGCCATGCAGGCCATGGGCGCCAGGATCCGTAAGGAA GGCGACACCTGGATCATCGATGGCGTCGGCAATGGCGGCCTCCTGGCGCCTGAGGCGCCGCTCGATTTCGGCAATGCCGCCACGGGCTGC CGCCTGACCATGGGCCTCGTCGGGGTCTACGATTTCGACAGCACCTTCATCGGCGACGCCTCGCTCACAAAGCGCCCGATGGGCCGCGTGT TGAACCCGCTGCGCGAAATGGGCGTGCAGGTGAAATCGGAAGACGGTGACCGTCTTCCCGTTACCTTGCGCGGGCCGA AGACGCCGACGC CGATCACCTACCGCGTGCCGATGGCCTCCGCACAGGTGAAGTCCGCCGTGCTGCTCGCCGGCCTCAACACGCCCGGCATCACGACGGTCAT CGAGCCGATCATGACGTGCGATCATACGGAAAAGATGCTGCAGGGCTTTGGCGCCAACCTTACCGTCGAGACGGATGCGGACGGCGTGC GCACCATCCGCCTGGAAGGCCGCGGCAAGCTCACCGGC CAAGTCATCGACGTGCCGGGCGACCCGTCCTCGACGGCCTTCCCGCTGGTTG CGGCCCTGCTTGTTCCGGGCTCCGACGTCACCATCCTCAACGTGCTGATGAACCCCACCCGCACCGGCCTCATCCTGACGCTGCAGGAAATG GGCGCCGACATCGAAGTCATCAACCTGCGCCTTGCCGGCGGCGAAGACGTGGCGGACCTGCGCGTTCGCTCCTCCACGCTGAAGGGCGTC ACGGTGCCGGAAGACCGCGCGCCTCCGATGATCGACGAATATCCGATT CTCGCTGTCGCCGCCGCCTTCGCGGAAGGGGCGACCGTGATG AACGGTCTGGAAGAACTCCGCGTCAAGGAAAGCGACCGCCTCTCGGCCGTCGCCAATGGCCTCAAGCTCAATGGCGTGGATTGCGATGAG GGCGAGACGTCGCTCGTCGTGCGTGGCCGCCCTGACGGCAAGGGGCTCGGCAACGCCTCGGGCGCCGCCGTCGCCACCCATCTCGATCAC CGCATCGCCATGAGCTTCCTCGTCATGGGCCTCG TGTCGGAAAACCCTGTCACGGTGGACGATGCCACGATGATCGCCACGAGCTTCCCGG AGTTCATGGACCTGATGGCCGGGCTGGGCGCGAAGATCGAACTCTCCGATACGAAGGCTGCC TGATGAGCTCGAATTCGAGCTCGGTACC GGATCCAATTCCCGATCGTTCAAACATTTGGCAATAAAGTTTCTTAAGATTGAATCCTGTTGCCGGTCTTGCGATGATTATCATATAATTTCT GTTGAATTACGTTAAGCATGTAATAATTAACATGTAATGCATGACGTTATTTATGAGATGGGTTTTTATGATTAGAGTCCCGCAATTATACAT TTAATACGCGATAGAAAACAAAATATAGCGCGCAAACT AGGATAAATTATCGCGCGCGGTGTCATCTATGTTACTAGATCGGGGATCGATC CCCCACCGGTCCTTCATGTTCGGCGGTCTCGCGAGCGGTGAAACGCGCATCACCGGCCTTCTGGAAGGCGAGGACGTCATCAATACGG GC AAGGCCATGCAGGCCATGGGCGCCAGGATCCGTAAGGAAGGCGACACCTGGATCATCGATGGCGTCGGCAATGGCGGCCTCCTGGCGCC TGAGGCGCCGCTCGATTTCGGCAATGCCGCCACGGGCTGCCGCCTGACCATGGGCCTCGTCGGGGTCTACGATTTCAAGCGCATCATGCTG GGAA
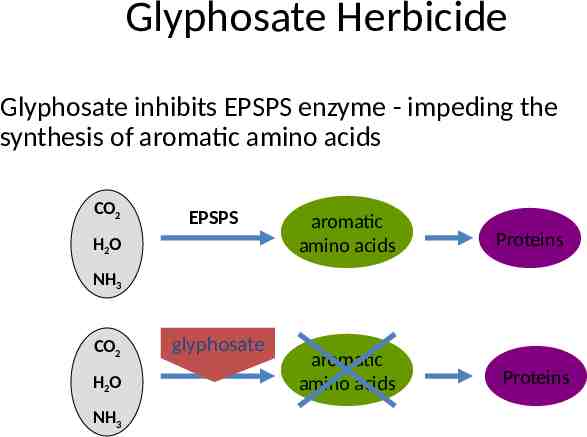
Glyphosate Herbicide Glyphosate inhibits EPSPS enzyme - impeding the synthesis of aromatic amino acids CO2 EPSPS H2O aromatic amino acids Proteins NH3 CO2 H2O NH3 glyphosate EPSPS aromatic amino acids Proteins

Commercial RR crops "Two key elements needed for the development of commercially viable glyphosate-tolerant crops are: – a resistant target enzyme – sufficient expression of that enzyme within the transgenic plant” Heck et al. 2005. Development and Characterization of a CP4 EPSPS-Based, Glyphosate-Tolerant Corn Event: Crop Sci. 45:329-339 (2005).
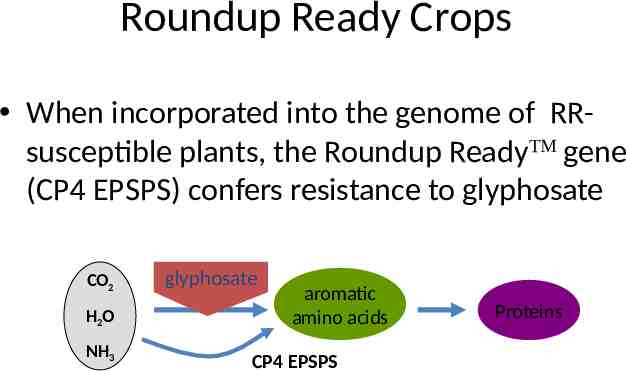
Roundup Ready Crops When incorporated into the genome of RRsusceptible plants, the Roundup ReadyTM gene (CP4 EPSPS) confers resistance to glyphosate CO2 H2O NH3 glyphosate EPSPS aromatic amino acids CP4 EPSPS Proteins

"Golden Rice” Creation of a biosynthetic pathway in rice using genes from daffodil and bacteria; portions of genes from pea, rice, and cauliflower mosaic virus

Golden Rice Vitamin A deficiency in humans a serious problem Rice is a major food crop Rice lacks provitamin A Create pathway for Beta-carotene synthesis Phytoene synthase from Narcissus pseudonarcissus Phytoene desaturase from Erwinia uredovora Endosperm-specific glutelin promoter from Oryza sativa CaMV promoter from cauliflower mosaic virus Transit peptide sequence from Pisum sativum www.forbes.com
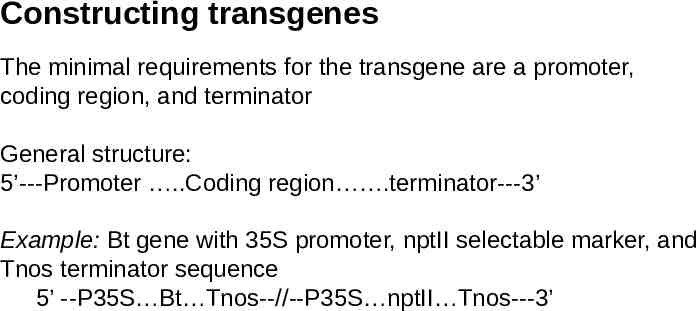
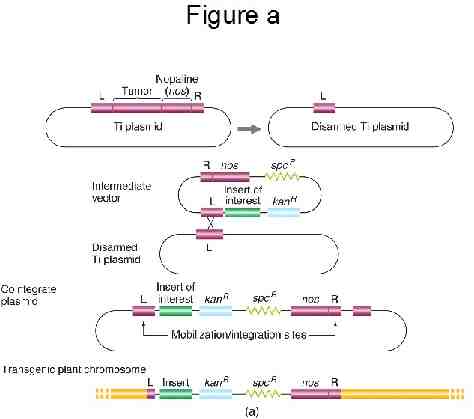
Constructing transgenes The minimal requirements for the transgene are a promoter, coding region, and terminator General structure: 5’---Promoter .Coding region .terminator---3’ Example: Bt gene with 35S promoter, nptII selectable marker, and Tnos terminator sequence 5’ --P35S Bt Tnos--//--P35S nptII Tnos---3’
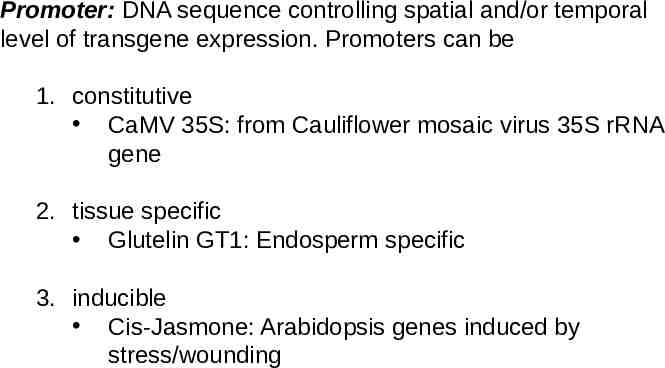
Promoter: DNA sequence controlling spatial and/or temporal level of transgene expression. Promoters can be 1. constitutive CaMV 35S: from Cauliflower mosaic virus 35S rRNA gene 2. tissue specific Glutelin GT1: Endosperm specific 3. inducible Cis-Jasmone: Arabidopsis genes induced by stress/wounding
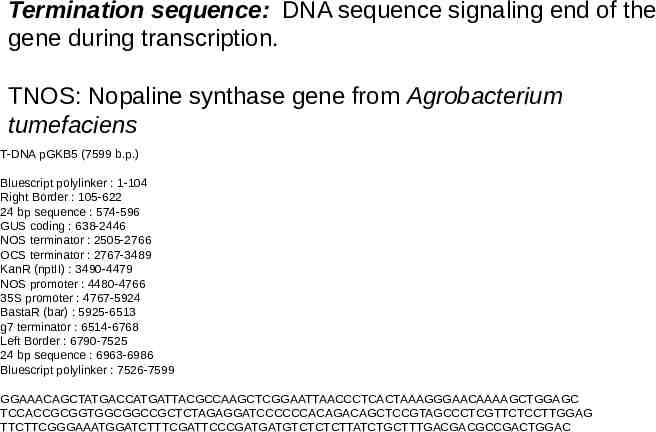
Termination sequence: DNA sequence signaling end of the gene during transcription. TNOS: Nopaline synthase gene from Agrobacterium tumefaciens T-DNA pGKB5 (7599 b.p.) Bluescript polylinker : 1-104 Right Border : 105-622 24 bp sequence : 574-596 GUS coding : 638-2446 NOS terminator : 2505-2766 OCS terminator : 2767-3489 KanR (nptII) : 3490-4479 NOS promoter : 4480-4766 35S promoter : 4767-5924 BastaR (bar) : 5925-6513 g7 terminator : 6514-6768 Left Border : 6790-7525 24 bp sequence : 6963-6986 Bluescript polylinker : 7526-7599 GGAAACAGCTATGACCATGATTACGCCAAGCTCGGAATTAACCCTCACTAAAGGGAACAAAAGCTGGAGC TCCACCGCGGTGGCGGCCGCTCTAGAGGATCCCCCCACAGACAGCTCCGTAGCCCTCGTTCTCCTTGGAG TTCTTCGGGAAATGGATCTTTCGATTCCCGATGATGTCTCTCTTATCTGCTTTGACGACGCCGACTGGAC

Selectable Marker: Encodes a protein (enzyme) that allows the transformed cells to grow while the growth of the nontransformed cells is inhibited. Examples include 1. Antibiotic resistance 2. Herbicide resistance “Among the most widely used antibiotic resistance genes as selectable markers are neomycin phosphotransferase II (nptII) and hygromycin phosphotransferase (hpt). The enzyme NPTII inactivates by phosphorylation a number of aminoglycoside antibiotics such as kanamycin, neomycin, geneticin (or G418) and paromomycin. Of these, G418 is routinely used for selection of transformed mammalian cells. The other three are used in a diverse range of plant species, however, kanamycin has proved to be ineffective to select legumes and gramineae. Hygromycin phosphotransferase is a suitable marker system for both plant and animal systems. The HPT enzyme inactivates the antibiotic hygromycin B. Hygromycin is usually more toxic than kanamycin and kills sensitive cells more quickly. It is nowadays one of the preferred antibiotic resistance marker systems for transformation of monocotyledonous plants, particularly gramineae (cereals and forages).” http://www.patentlens.net/daisy/Antibiotic/g1/1155.html
Selectable Marker: Encodes a protein (enzyme) that allows the transformed cells to grow while the growth of the nontransformed cells is inhibited. Examples include 2. Herbicide resistance “.Members of the genus Streptomyces produce bialaphos, which leads to the production of glufosiante, ultimately inhibiting glutamine synthetase. The biochemical and toxicological characteristics of glufosinate have made it a popular, nonselective herbicide, which has been commercialized under the names Basta , Buster and Liberty by Bayer Crop Science. Other Streptomyces spp. can detoxify glufosinate by producing an acetylating enzyme via production of a phosphinothricin acetyl transferase (PAT) enzyme encoded by the bar (bialaphos resistance) gene. Treatment of genetically modified plants carrying a bar gene with glufosinate or bialaphos provides a very efficient means of selection in genetic transformation protocols.” http://www.patentlens.net/daisy/Phosph/g2/710.html
Selectable Markers. Details and examples of selectable markers
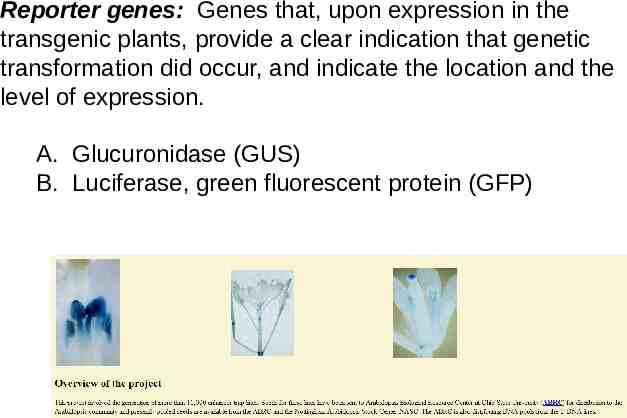
Reporter genes: Genes that, upon expression in the transgenic plants, provide a clear indication that genetic transformation did occur, and indicate the location and the level of expression. A. Glucuronidase (GUS) B. Luciferase, green fluorescent protein (GFP)
GFP: So many ways to be green
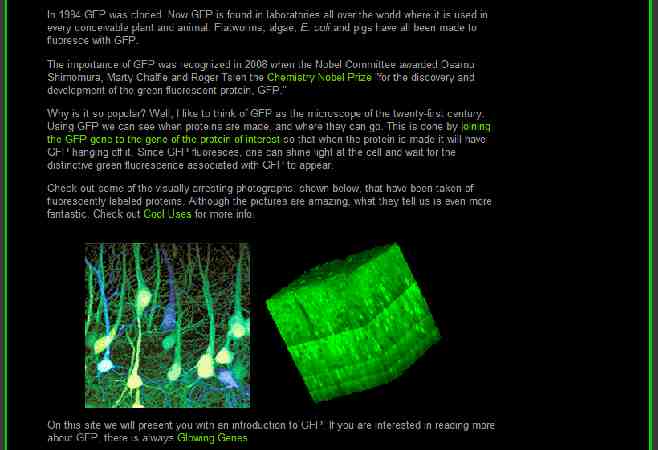
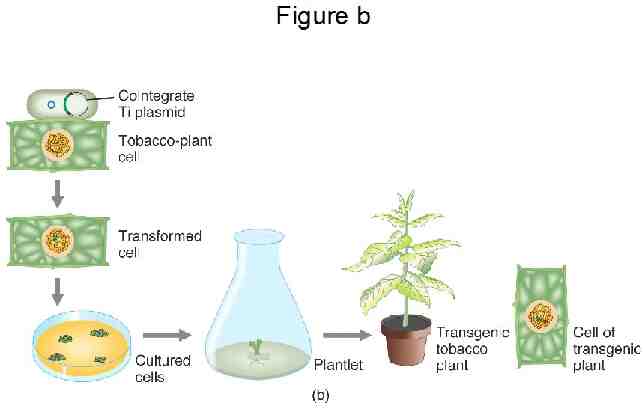
Transformation procedures: In order for a transgene to be inherited, it must be incorporated into the genome of a cell which will give rise to tissues which will be asexually propagated or to tissues which will undergo gametogenesis The two principal mechanisms for transforming tissues with a transgene Biolistics “Gene gun” Agrobacterium tumefaciens “Agro”
The gene gun Micro projectile bombardment or the biolistic method Small metal particles are coated with the transgene DNA Particles are delivered to target tissues via an explosive force “The Helios Gene Gun is a new way for in vivo transformation of cells or organisms apy and genetic immunization (DNA vaccination)). This gun uses Biolistic particle bombardment where DNA- or RNA-coated gold particles are loaded into the gun and you pull the trigger. A low pressure helium pulse delivers the coated gold particles into virtually any target cell or tissue. The particles carry the DNA so that you do not have to remove cells from tissue in order to transform the cells.”

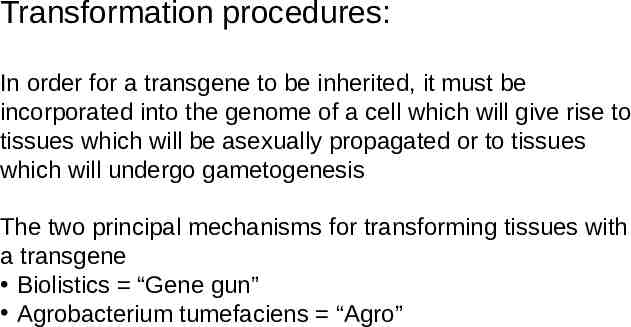
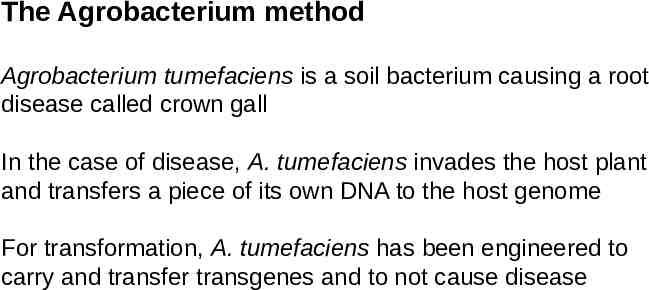
The Agrobacterium method Agrobacterium tumefaciens is a soil bacterium causing a root disease called crown gall In the case of disease, A. tumefaciens invades the host plant and transfers a piece of its own DNA to the host genome For transformation, A. tumefaciens has been engineered to carry and transfer transgenes and to not cause disease
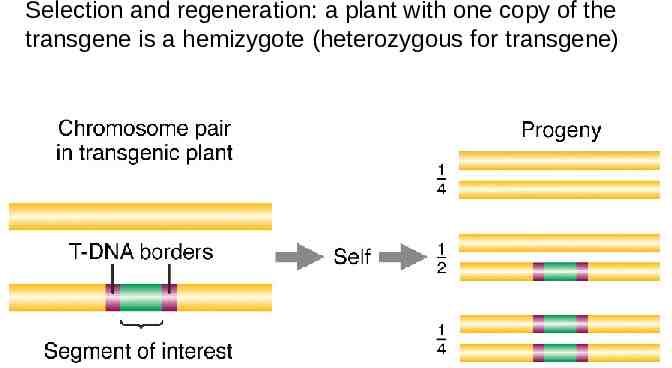
Selection and regeneration: a plant with one copy of the transgene is a hemizygote (heterozygous for transgene)
Concerns regarding transgenic plants Cultural/ religious issues - e.g. animal genes in plants Dietary concerns - e.g. allergies to novel proteins Gene escape - e.g. RoundUp Ready beets Non-target organisms - e.g. transgene plant products/ parts with unanticipated consequences Wasting precious genes one at a time - e.g. widespread use of single Bt genes could provide intense selection pressure for resistant insects, rendering the use of Bt spray ineffective Ownership - e.g. transgene technologies, and genes, are generally subject to intellectual property protection
Bringing it all home .to Oregon Herbicide resistant bentgrass in Oregon (For the Willamette Valley sugarbeet story, see Lecture 1)
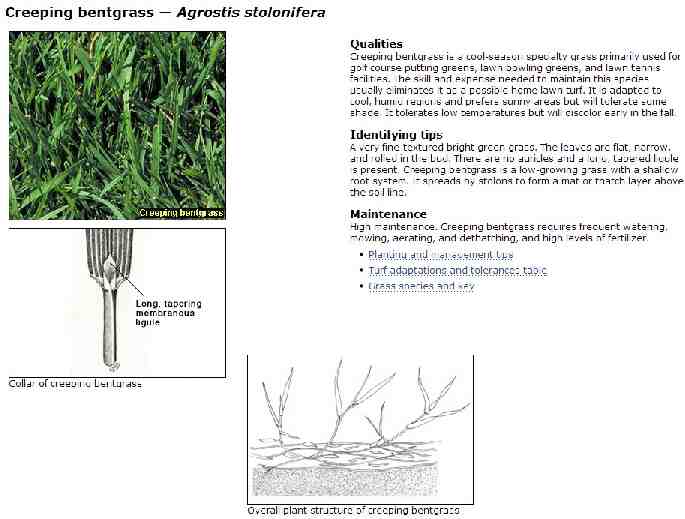
The plant: Creeping bent grass (Agrostis stolonifera L.) Golf greens Considered a weed in eight countries Other Agrostis species (250 worldwide; 34 North American; 24 native) Diploid to polyploid Sexual propagation Outcrossing A. stolonifera is obligate outcrossing Small seeds Asexual propagation stolons rhizomes
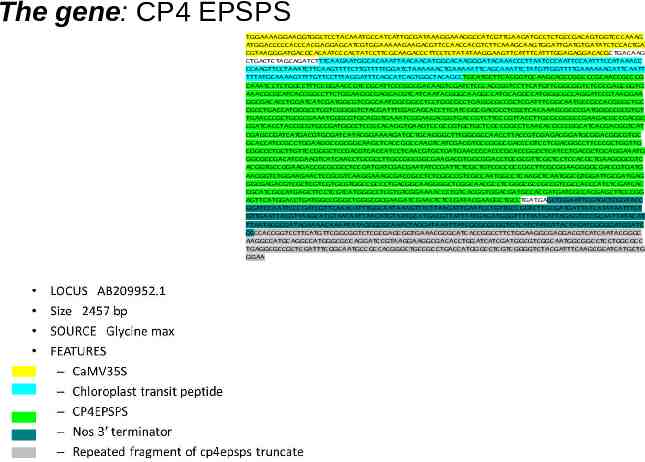
The gene: CP4 EPSPS TGGAAAAGGAAGGTGGCTCCTACAAATGCCATCATTGCGATAAAGGAAAGGCCATCGTTGAAGATGCCTCTGCCGACAGTGGTCCCAAAG ATGGACCCCCACCCACGAGGAGCATCGTGGAAAAAGAAGACGTTCCAACCACGTCTTCAAAGCAAGTGGATTGATGTGATATCTCCACTGA CGTAAGGGATGACGCACAATCCCACTATCCTTCGCAAGACCCTTCCTCTATATAAGGAAGTTCATTTCATTTGGAGAGGACACGCTGACAAG CTGACTCTAGCAGATCTTTCAAGAATGGCACAAATTAACAACATGGCACAAGGGATACAAACCCTTAATCCCAATTCCAATTTCCATAAACC CCAAGTTCCTAAATCTTCAAGTTTTCTTGTTTTTGGATCTAAAAAACTGAAAAATTCAGCAAATTCTATGTTGGTTTTGAAAAAAGATTCAATT TTTATGCAAAAGTTTTGTTCCTTTAGGATTTCAGCATCAGTGGCTACAGCCTGCATGCTTCACGGTGCAAGCAGCCGGCCCGCAACCGCCCG CAAATCCTCTGGCCTTTCCGGAACCGTCCGCATTCCCGGCGACAAGTCGATCTCCCACCGGTCCTTCATGTTCGGCGGTCTCGCGAGCGGTG AAACGCGCATCACCGGCCTTCTGGAAGGCGAGGACGTCATCAATACGGGCAAGGCCATGCAGGCCATGGGCGCCAGGATCCGTAAGGAA GGCGACACCTGGATCATCGATGGCGTCGGCAATGGCGGCCTCCTGGCGCCTGAGGCGCCGCTCGATTTCGGCAATGCCGCCACGGGCTGC CGCCTGACCATGGGCCTCGTCGGGGTCTACGATTTCGACAGCACCTTCATCGGCGACGCCTCGCTCACAAAGCGCCCGATGGGCCGCGTGT TGAACCCGCTGCGCGAAATGGGCGTGCAGGTGAAATCGGAAGACGGTGACCGTCTTCCCGTTACCTTGCGCGGGCCGAAGACGCCGACGC CGATCACCTACCGCGTGCCGATGGCCTCCGCACAGGTGAAGTCCGCCGTGCTGCTCGCCGGCCTCAACACGCCCGGCATCACGACGGTCAT CGAGCCGATCATGACGTGCGATCATACGGAAAAGATGCTGCAGGGCTTTGGCGCCAACCTTACCGTCGAGACGGATGCGGACGGCGTGC GCACCATCCGCCTGGAAGGCCGCGGCAAGCTCACCGGCCAAGTCATCGACGTGCCGGGCGACCCGTCCTCGACGGCCTTCCCGCTGGTTG CGGCCCTGCTTGTTCCGGGCTCCGACGTCACCATCCTCAACGTGCTGATGAACCCCACCCGCACCGGCCTCATCCTGACGCTGCAGGAAATG GGCGCCGACATCGAAGTCATCAACCTGCGCCTTGCCGGCGGCGAAGACGTGGCGGACCTGCGCGTTCGCTCCTCCACGCTGAAGGGCGTC ACGGTGCCGGAAGACCGCGCGCCTCCGATGATCGACGAATATCCGATTCTCGCTGTCGCCGCCGCCTTCGCGGAAGGGGCGACCGTGATG AACGGTCTGGAAGAACTCCGCGTCAAGGAAAGCGACCGCCTCTCGGCCGTCGCCAATGGCCTCAAGCTCAATGGCGTGGATTGCGATGAG GGCGAGACGTCGCTCGTCGTGCGTGGCCGCCCTGACGGCAAGGGGCTCGGCAACGCCTCGGGCGCCGCCGTCGCCACCCATCTCGATCAC CGCATCGCCATGAGCTTCCTCGTCATGGGCCTCGTGTCGGAAAACCCTGTCACGGTGGACGATGCCACGATGATCGCCACGAGCTTCCCGG AGTTCATGGACCTGATGGCCGGGCTGGGCGCGAAGATCGAACTCTCCGATACGAAGGCTGCCTGATGAGCTCGAATTCGAGCTCGGTACC GGATCCAATTCCCGATCGTTCAAACATTTGGCAATAAAGTTTCTTAAGATTGAATCCTGTTGCCGGTCTTGCGATGATTATCATATAATTTCT GTTGAATTACGTTAAGCATGTAATAATTAACATGTAATGCATGACGTTATTTATGAGATGGGTTTTTATGATTAGAGTCCCGCAATTATACAT TTAATACGCGATAGAAAACAAAATATAGCGCGCAAACTAGGATAAATTATCGCGCGCGGTGTCATCTATGTTACTAGATCGGGGATCGATC CCCCACCGGTCCTTCATGTTCGGCGGTCTCGCGAGCGGTGAAACGCGCATCACCGGCCTTCTGGAAGGCGAGGACGTCATCAATACGGGC AAGGCCATGCAGGCCATGGGCGCCAGGATCCGTAAGGAAGGCGACACCTGGATCATCGATGGCGTCGGCAATGGCGGCCTCCTGGCGCC TGAGGCGCCGCTCGATTTCGGCAATGCCGCCACGGGCTGCCGCCTGACCATGGGCCTCGTCGGGGTCTACGATTTCAAGCGCATCATGCTG GGAA
The case: 2003 Under APHIS permit, 162 ha test in Jefferson county of glyphosatetolerant GM creeping bentgrass (event ASR368 by Scotts and Monsanto). The test was conducted within a 4453-ha control area established by the Oregon Dept. of Agriculture. 2004: 2.5 ha of production. Documented gene flow primarily within 2 km but up to 21 km (Watrud et al. 2004. PNAS 14533-14538) Gene flow documented via seedling tests using protein detection (TraitCheck) PCR (using transgenic soybean CP4 EPSPS; GenBank Accession No. AF464188.1, sequencing of cloned PCR products.
The case: 2005: No production Continued sampling 2006: Detected plants expressing transgene - demonstrated pollen transfer and seed dispersal (Reichman et al. 2006. Mol. Ecology 15: 42434255) Gene flow documented via using TraitChek, PCR, and sequencing 2007: Issue settled with civil penalty 2011: Transgenic plants in central and southeastern Oregon 2012: Transgenic plants in Oregon and Idaho 2013, 2014, 2015, 2016, 2017 monitoring and reporting

What is and what is not a transgenic plant?
“Our work suggests that cisgenic insertion of additional copies of native genes involved in growth regulation may provide tools to help modify plant architecture, expand the genetic variance in plant architecture available to breeders and accelerate transfer of alleles between difficult-to-cross species.”
http://www.nature.com/nrg/multimedia/rnai/animation/index.html

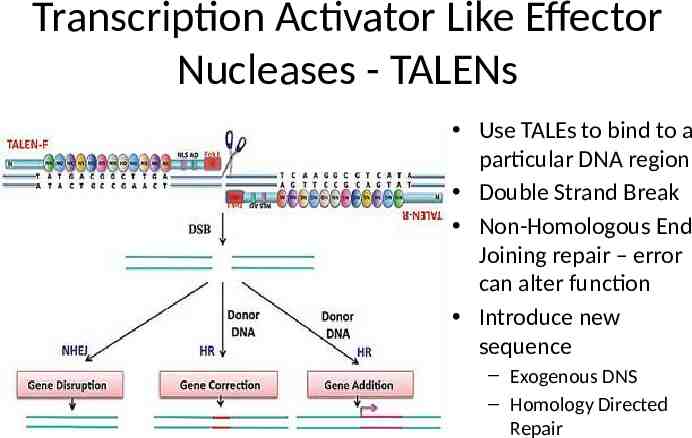
Transcription Activator Like Effector Nucleases - TALENs Use TALEs to bind to a particular DNA region Double Strand Break Non-Homologous End Joining repair – error can alter function Introduce new sequence – Exogenous DNS – Homology Directed Repair

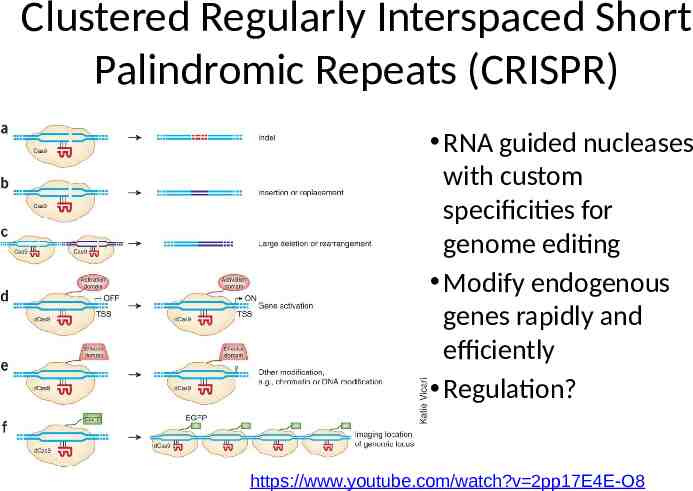
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) RNA guided nucleases with custom specificities for genome editing Modify endogenous genes rapidly and efficiently Regulation? https://www.youtube.com/watch?v 2pp17E4E-O8

Genome Editing Example
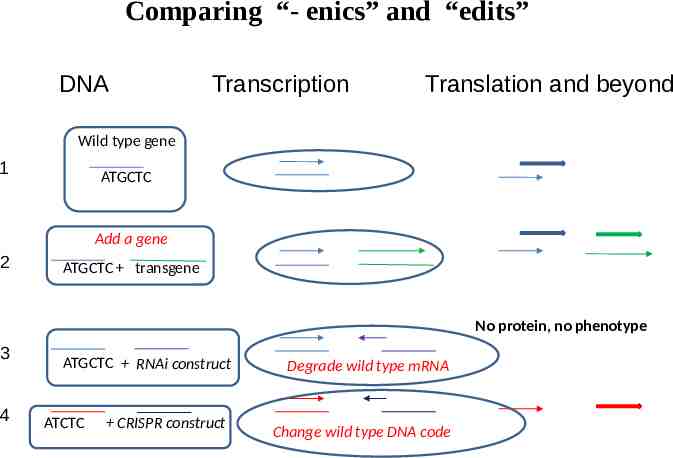
Comparing “- enics” and “edits” DNA Transcription Translation and beyond Wild type gene 1 ATGCTC Add a gene 2 ATGCTC transgene No protein, no phenotype 3 4 ATGCTC RNAi construct ATCTC CRISPR construct Degrade wild type mRNA Change wild type DNA code