Definitive and Postoperative Radiation Therapy for Basal and Squamous
44 Slides442.94 KB

Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: An ASTRO Clinical Practice Guideline Developed in Collaboration with the American Society of Clinical Oncology and Society of Surgical Oncology Endorsed by the American Association of Physicists in Medicine, American Brachytherapy Society, American College of Radiology, American Head and Neck Society, and the Society of Surgical Oncology

Citation This slide set is adapted from the Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin Guideline, published in the January/February 2020 issue of Practical Radiation Oncology (PRO). The guideline was e-published ( https://doi.org/10.1016/j.prro.2019.10.014) on December 9, 2019, and is also available on the ASTRO website: www.astro.org

Guideline Task Force Chairs: Phillip M. Devlin, MD Anna Likhacheva, MD, MPH Members: Musaddiq Awan, MD Christopher A. Barker, MD Ajay Bhatnagar, MD Lisa Bradfield Mary Sue Brady, MD Ivan Buzurovic, PhD Jessica L. Geiger, MD Upendra Parvathaneni, MBBS Sandra Zaky, MD

Task Force Composition Radiation oncology o Drawn from academic practice, private or community practice, and the Veterans Health Administration system o Include a RO resident and a member of the Guidelines Subcommittee Related specialties/disciplines* o radiation, medical, and surgical oncologists o Medical physicist *Non-RO physicians are nominated by their respective societies Patient representative
Guideline Scope To review the evidence and provide recommendations for the use of definitive and postoperative radiation therapy (RT) in patients with basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) as well as dose-fractionation schemes, target volumes, basic aspects of treatment planning, choice of radiation modality and the role of systemic therapy in combination with radiation.

Systematic Review MEDLINE PubMed - 5/01/1988 – 6/31/2018 – Both MeSH terms and text words used and then supplemented with hand searches – Outcomes: local and regional recurrence risk, disease-free survival, overall survival, toxicity, quality of life (QoL) Inclusion: Age 18 years, diagnosis of nonmetastatic invasive BCC and cSCC, RT delivered with curative intent. – KQ1 included studies with 100 patients, KQs 2-4 used 50 patients and KQ5 reduced the patient number to 15 since minimal evidence exists on chemotherapy, biologic, and immunotherapy agents Exclusion: metastatic BCC and cSCC; dermatopathologic aspects of diagnosis, surgical nuances, technical details of RT, mucosal head and neck SCC, vulvar, penile, and perianal skin carcinoma; preclinical and dosimetric studies, publications addressing re-irradiation or palliation, non-English, case reports, not relevant to KQs 1515 citations identified 193 articles assessed 143 articles included and abstracted into evidence tables
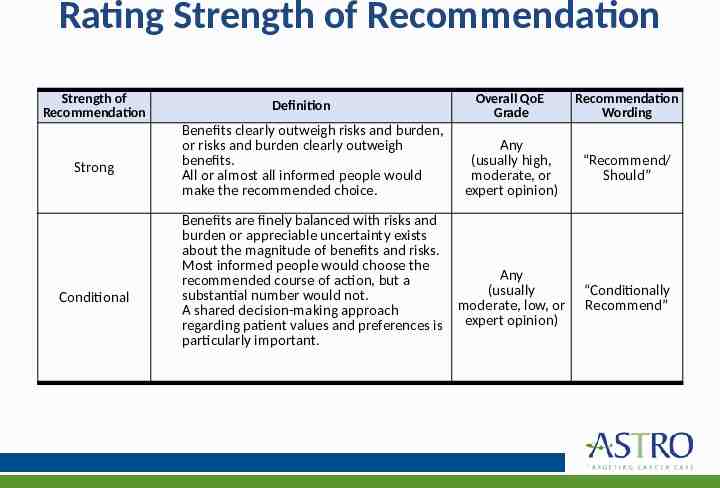
Rating Strength of Recommendation Strength of Recommendation Strong Conditional Definition Benefits clearly outweigh risks and burden, or risks and burden clearly outweigh benefits. All or almost all informed people would make the recommended choice. Overall QoE Grade Recommendation Wording Any (usually high, moderate, or expert opinion) “Recommend/ Should” Benefits are finely balanced with risks and burden or appreciable uncertainty exists about the magnitude of benefits and risks. Most informed people would choose the Any recommended course of action, but a (usually substantial number would not. moderate, low, or A shared decision-making approach regarding patient values and preferences is expert opinion) particularly important. “Conditionally Recommend”
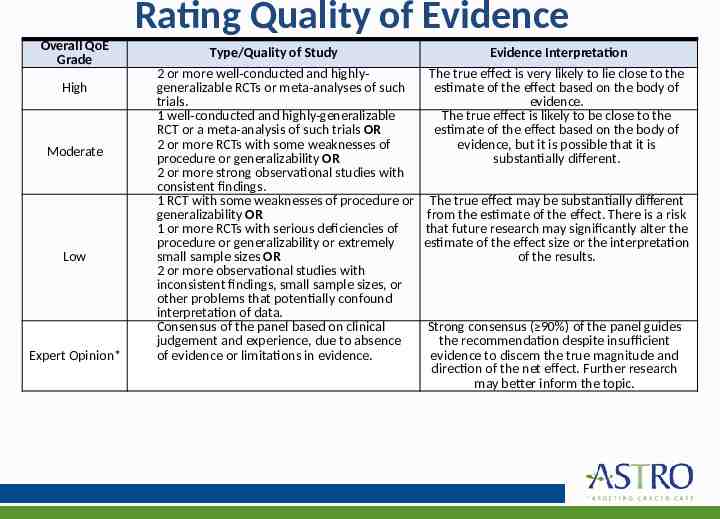
Rating Quality of Evidence Overall QoE Grade Type/Quality of Study 2 or more well-conducted and highlygeneralizable RCTs or meta-analyses of such trials. 1 well-conducted and highly-generalizable RCT or a meta-analysis of such trials OR 2 or more RCTs with some weaknesses of Moderate procedure or generalizability OR 2 or more strong observational studies with consistent findings. 1 RCT with some weaknesses of procedure or generalizability OR 1 or more RCTs with serious deficiencies of procedure or generalizability or extremely small sample sizes OR Low 2 or more observational studies with inconsistent findings, small sample sizes, or other problems that potentially confound interpretation of data. Consensus of the panel based on clinical judgement and experience, due to absence of evidence or limitations in evidence. Expert Opinion* High Evidence Interpretation The true effect is very likely to lie close to the estimate of the effect based on the body of evidence. The true effect is likely to be close to the estimate of the effect based on the body of evidence, but it is possible that it is substantially different. The true effect may be substantially different from the estimate of the effect. There is a risk that future research may significantly alter the estimate of the effect size or the interpretation of the results. Strong consensus ( 90%) of the panel guides the recommendation despite insufficient evidence to discern the true magnitude and direction of the net effect. Further research may better inform the topic.

Consensus Methodology Modified Delphi approach Task force members rated their agreement with each recommendation using an online consensus survey – 5-point Likert scale from “strongly disagree” to “strongly agree” – Consensus defined using pre-specified threshold of 75% ( 90% for expert opinion recommendations) agreement Recommendations for which consensus is not achieved are removed or are revised and re-surveyed. Recommendations achieving consensus edited with substantive changes after the first round are also resurveyed.

KQ 1: What are the appropriate indications for definitive RT for BCC and cSCC? KQ1 Recommendations 1. In patients with BCC and cSCC who cannot undergo or decline surgical resection, definitive RT is recommended as a curative treatment modality. Strength of Quality of Recommendation Evidence Strong Moderate
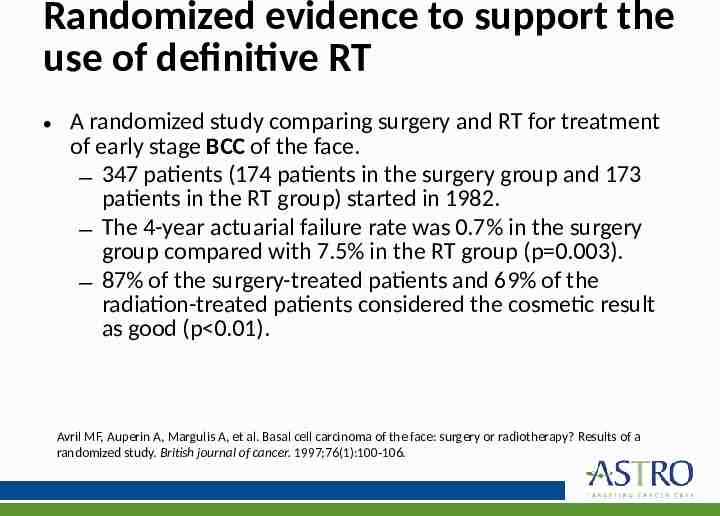
Randomized evidence to support the use of definitive RT A randomized study comparing surgery and RT for treatment of early stage BCC of the face. – 347 patients (174 patients in the surgery group and 173 patients in the RT group) started in 1982. – The 4-year actuarial failure rate was 0.7% in the surgery group compared with 7.5% in the RT group (p 0.003). – 87% of the surgery-treated patients and 69% of the radiation-treated patients considered the cosmetic result as good (p 0.01). Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. British journal of cancer. 1997;76(1):100-106.
Evidence to support the use of definitive RT for cSCC and BCC Retrospective and single-arm prospective evidence characterizing outcomes for skin carcinomas after treatment with modern RT: – A meta-analysis of 9729 patients (21 studies) with BCC and cSCC confirmed excellent control with RT.1 Median 1-year LR rate was 2% and the 5-year LR rate was 14% when combining all fractionation regimens. – A meta-analysis of 40 randomized and 5 nonrandomized studies of various available interventions for primary cutaneous BCC.2 LR rates were similar for excision (3.8%), Mohs surgery (3.8%), and EBRT(3.5%). LR rates for cryotherapy (22.3%) curettage and cryotherapy (19.9%), 5fluorouracil (18.8%), imiquimod (14.1%), and photodynamic therapy using methyl-aminolevulinic acid (18.8) or aminolevulinic acid (16.6). 1. Zaorsky NG, Lee CT, Zhang E, Keith SW, Galloway TJ. Hypofractionated radiation therapy for basal and squamous cell skin cancer: A meta-analysis. Radiotherapy & Oncology. 2017;125:13-20. 2. Drucker AM, Adam GP, Rofeberg V, et al. Treatments of Primary Basal Cell Carcinoma of the Skin: A Systematic Review and Network Meta-analysis. Ann Intern Med. 2018;169(7):456-466.
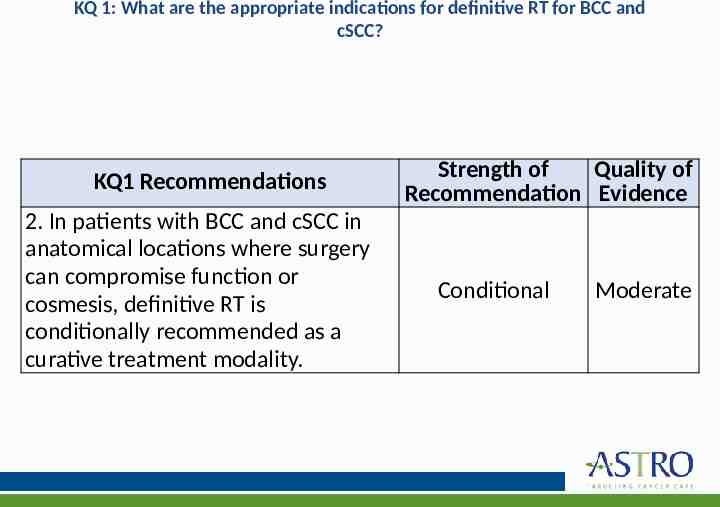
KQ 1: What are the appropriate indications for definitive RT for BCC and cSCC? KQ1 Recommendations 2. In patients with BCC and cSCC in anatomical locations where surgery can compromise function or cosmesis, definitive RT is conditionally recommended as a curative treatment modality. Strength of Quality of Recommendation Evidence Conditional Moderate
Cosmetic and functional aspect of definitive RT for BCC and cSCC Good functional outcomes are especially relevant for commonly sun exposed area of the face where surgical deformity can cause decreased QoL. – Nose – Lips – Eyelids – Ears

Cosmetic and functional aspect of definitive RT for BCC and cSCC Zaorsky meta-analysis found “good” or “better” cosmesis in the 21 studies to be 80% at 5 years. Single arm studies reporting excellent functional preservation in – Peri-orbital targets – Lip – Nose Zaorsky NG, Lee CT, Zhang E, Keith SW, Galloway TJ. Hypofractionated radiation therapy for basal and squamous cell skin cancer: A meta-analysis. Radiotherapy & Oncology. 2017;125:13-20. de Visscher JG, Botke G, Schakenraad JA, van der Waal I. A comparison of results after radiotherapy and surgery for stage I squamous cell carcinoma of the lower lip. Head & neck. 1999;21(6):526-530. Mazeron JJ, Chassagne D, Crook J, et al. Radiation therapy of carcinomas of the skin of nose and nasal vestibule: a report of 1676 cases by the Groupe Europeen de Curietherapie. Radiotherapy & Oncology. 1988;13(3):165-173. Krengli M, Masini L, Comoli AM, et al. Interstitial brachytherapy for eyelid carcinoma. Outcome analysis in 60 patients. Strahlentherapie und Onkologie. 2014;190:245-249.
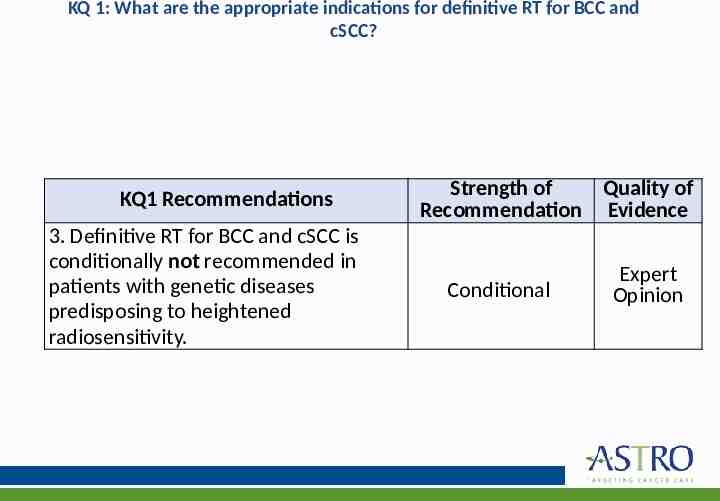
KQ 1: What are the appropriate indications for definitive RT for BCC and cSCC? KQ1 Recommendations 3. Definitive RT for BCC and cSCC is conditionally not recommended in patients with genetic diseases predisposing to heightened radiosensitivity. Strength of Quality of Recommendation Evidence Conditional Expert Opinion
Contraindications to definitive RT The use of definitive RT is discouraged for the treatment of cSCC or BCC in patients with genetic conditions predisposing to heightened radiosensitivity, such as ataxia telangiectasia, nevoid basal cell carcinoma syndrome (Gorlin Syndrome) and Li Fraumeni syndrome. Poorly controlled connective tissue disorders are a relative contraindication to treatment. Overall life expectancy should be considered and discussed with younger patients, for whom a larger lifetime risk of developing secondary malignancy in the treatment field is expected. Baker S, Joseph K, Tai P. Radiotherapy in Gorlin Syndrome: Can It Be Safe and Effective in Adult Patients? Journal of cutaneous medicine and surgery. 2016;20(2):159-162. Martin F. Lavin. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nature Reviews Molecular Cell Biology volume 9, pages 759–769 (2008) Heymann, et al. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol. 2010 Nov 8;5:104. doi: 10.1186/1748-717X-5-104.
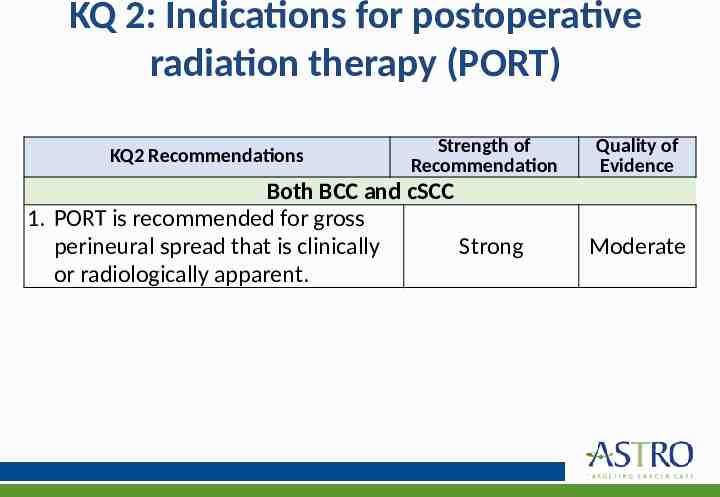
KQ 2: Indications for postoperative radiation therapy (PORT) KQ2 Recommendations Strength of Recommendation Both BCC and cSCC 1. PORT is recommended for gross perineural spread that is clinically Strong or radiologically apparent. Quality of Evidence Moderate

Indications for PORT in cSCC Cutaneous SCC is a much more aggressive entity than BCC with a far greater risk for regional and nodal spread. Thus, the task force recommends more wide-ranging utilization of PORT in the SCC population. Lin C, Tripcony L, Keller J, Poulsen M, Dickie G. Cutaneous carcinoma of the head and neck with clinical features of perineural infiltration treated with radiotherapy. Clinical oncology (Royal College of Radiologists (Great Britain)). 2013;25(6):362-367. Jackson JE, Dickie GJ, Wiltshire KL, et al. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: toward a risk-adapted treatment approach. Head & neck. 2009;31(5):604-610.
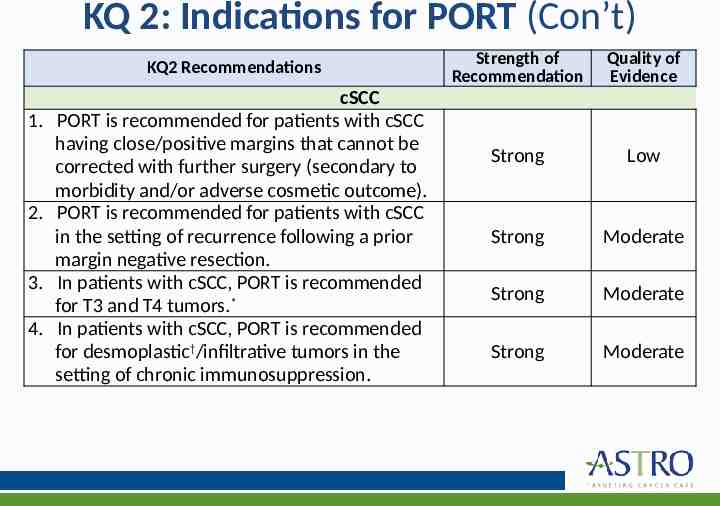
KQ 2: Indications for PORT (Con’t) KQ2 Recommendations 1. 2. 3. 4. cSCC PORT is recommended for patients with cSCC having close/positive margins that cannot be corrected with further surgery (secondary to morbidity and/or adverse cosmetic outcome). PORT is recommended for patients with cSCC in the setting of recurrence following a prior margin negative resection. In patients with cSCC, PORT is recommended for T3 and T4 tumors.* In patients with cSCC, PORT is recommended for desmoplastic†/infiltrative tumors in the setting of chronic immunosuppression. Strength of Recommendation Quality of Evidence Strong Low Strong Moderate Strong Moderate Strong Moderate
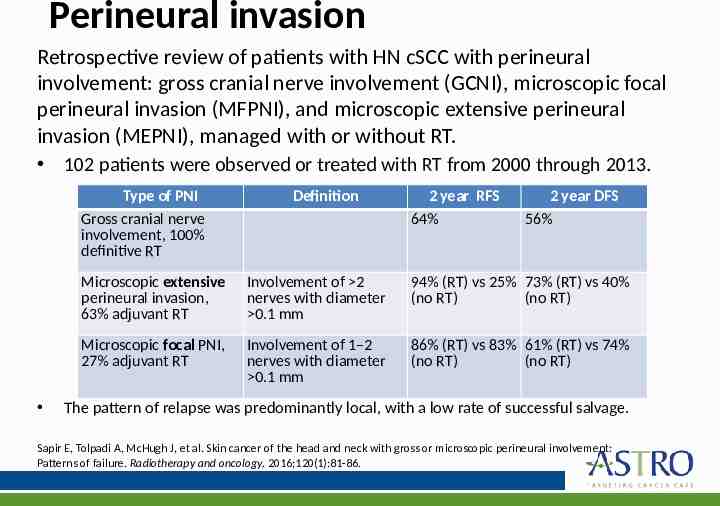
Perineural invasion Retrospective review of patients with HN cSCC with perineural involvement: gross cranial nerve involvement (GCNI), microscopic focal perineural invasion (MFPNI), and microscopic extensive perineural invasion (MEPNI), managed with or without RT. 102 patients were observed or treated with RT from 2000 through 2013. Type of PNI Gross cranial nerve involvement, 100% definitive RT Definition 2 year RFS 64% 2 year DFS 56% Microscopic extensive perineural invasion, 63% adjuvant RT Involvement of 2 nerves with diameter 0.1 mm 94% (RT) vs 25% 73% (RT) vs 40% (no RT) (no RT) Microscopic focal PNI, 27% adjuvant RT Involvement of 1–2 nerves with diameter 0.1 mm 86% (RT) vs 83% 61% (RT) vs 74% (no RT) (no RT) The pattern of relapse was predominantly local, with a low rate of successful salvage. Sapir E, Tolpadi A, McHugh J, et al. Skin cancer of the head and neck with gross or microscopic perineural involvement: Patterns of failure. Radiotherapy and oncology. 2016;120(1):81-86.

Other high-risk features for recurrence Retrospective analysis of stage I through IV head and neck cSCC who underwent surgery and also received PORT for primary or recurrent disease. 205 patients treated between 1995 and 2015 – On MVA, immunosuppressed status (hazard ratio [HR]: 3.79), recurrent disease (HR: 2.67), poor differentiation (HR: 2.08), and PNI (HR: 2.05) were significantly associated with locoregional recurrence Manyam BV, Garsa AA, Chin RI, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer. 2017;123(11):2054-2060.
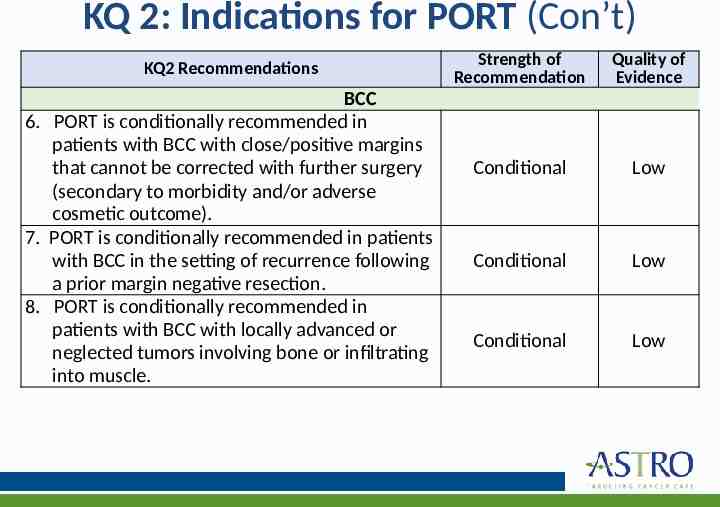
KQ 2: Indications for PORT (Con’t) KQ2 Recommendations BCC 6. PORT is conditionally recommended in patients with BCC with close/positive margins that cannot be corrected with further surgery (secondary to morbidity and/or adverse cosmetic outcome). 7. PORT is conditionally recommended in patients with BCC in the setting of recurrence following a prior margin negative resection. 8. PORT is conditionally recommended in patients with BCC with locally advanced or neglected tumors involving bone or infiltrating into muscle. Strength of Recommendation Quality of Evidence Conditional Low Conditional Low Conditional Low
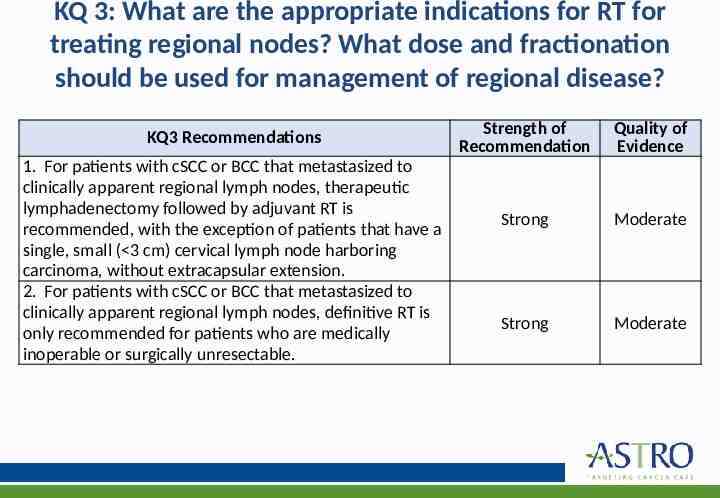
KQ 3: What are the appropriate indications for RT for treating regional nodes? What dose and fractionation should be used for management of regional disease? KQ3 Recommendations 1. For patients with cSCC or BCC that metastasized to clinically apparent regional lymph nodes, therapeutic lymphadenectomy followed by adjuvant RT is recommended, with the exception of patients that have a single, small ( 3 cm) cervical lymph node harboring carcinoma, without extracapsular extension. 2. For patients with cSCC or BCC that metastasized to clinically apparent regional lymph nodes, definitive RT is only recommended for patients who are medically inoperable or surgically unresectable. Strength of Recommendation Quality of Evidence Strong Moderate Strong Moderate
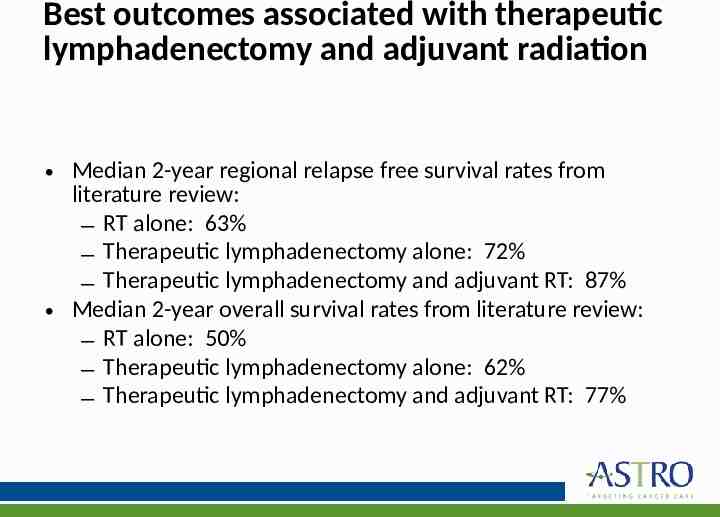
Best outcomes associated with therapeutic lymphadenectomy and adjuvant radiation Median 2-year regional relapse free survival rates from literature review: – RT alone: 63% – Therapeutic lymphadenectomy alone: 72% – Therapeutic lymphadenectomy and adjuvant RT: 87% Median 2-year overall survival rates from literature review: – RT alone: 50% – Therapeutic lymphadenectomy alone: 62% – Therapeutic lymphadenectomy and adjuvant RT: 77%

Best outcomes associated with therapeutic lymphadenectomy and adjuvant radiation However, in medically inoperable or surgically unresectable nodal metastases, radiation therapy is recommended In addition, patients with a single, small ( 3 cm) cervical lymph node were found to be at low risk for regional recurrence after therapeutic lymphadenectomy alone, and may not need RT

KQ 3: What are the appropriate indications for RT for treating regional nodes? What dose and fractionation should be used for management of regional disease? KQ3 Recommendations 3. For patients with cSCC at high risk of regional nodal metastasis, imaging and sentinel lymph node biopsy are conditionally recommended to guide the need for and target of lymph node basin RT. Implementation Remark: Close clinical follow-up of the lymph node basin is important for patients in whom sentinel lymph node biopsy is unlikely to be accurate due to: 1) an extensive initial primary resection and/or reconstruction or 2) tumor location in the head and neck area. 4. For patients with cSCC at high risk of regional nodal metastasis (thickness 6 mm), elective lymph node basin RT is conditionally recommended only for those undergoing RT to the primary site with overlap of the adjacent nodal basin. Strength of Quality of Recommendation Evidence Conditional Expert Opinion Conditional Low
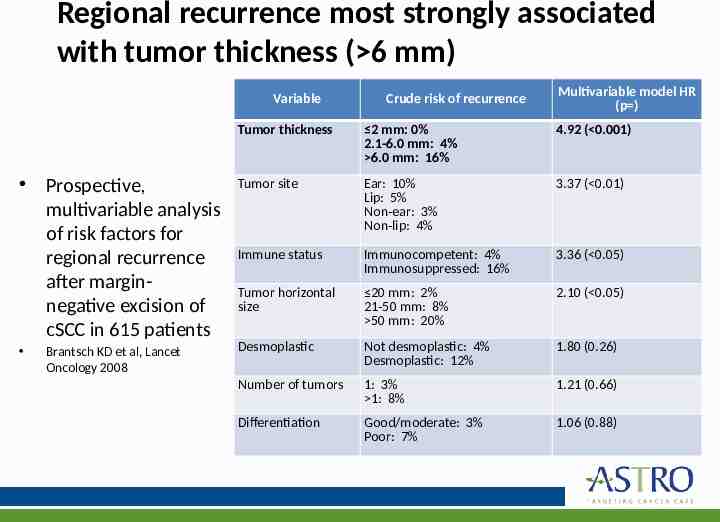
Regional recurrence most strongly associated with tumor thickness ( 6 mm) Variable Crude risk of recurrence Multivariable model HR (p ) Tumor thickness 2 mm: 0% 2.1-6.0 mm: 4% 6.0 mm: 16% 4.92 ( 0.001) Prospective, multivariable analysis of risk factors for regional recurrence after marginnegative excision of cSCC in 615 patients Tumor site Ear: 10% Lip: 5% Non-ear: 3% Non-lip: 4% 3.37 ( 0.01) Immune status Immunocompetent: 4% Immunosuppressed: 16% 3.36 ( 0.05) Tumor horizontal size 20 mm: 2% 21-50 mm: 8% 50 mm: 20% 2.10 ( 0.05) Desmoplastic Not desmoplastic: 4% Desmoplastic: 12% 1.80 (0.26) Number of tumors 1: 3% 1: 8% 1.21 (0.66) Differentiation Good/moderate: 3% Poor: 7% 1.06 (0.88) Brantsch KD et al, Lancet Oncology 2008
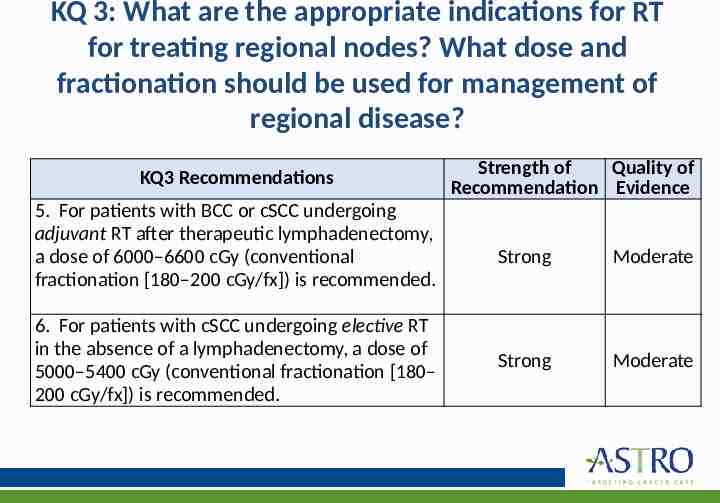
KQ 3: What are the appropriate indications for RT for treating regional nodes? What dose and fractionation should be used for management of regional disease? KQ3 Recommendations 5. For patients with BCC or cSCC undergoing adjuvant RT after therapeutic lymphadenectomy, a dose of 6000–6600 cGy (conventional fractionation [180–200 cGy/fx]) is recommended. 6. For patients with cSCC undergoing elective RT in the absence of a lymphadenectomy, a dose of 5000–5400 cGy (conventional fractionation [180– 200 cGy/fx]) is recommended. Strength of Quality of Recommendation Evidence Strong Moderate Strong Moderate
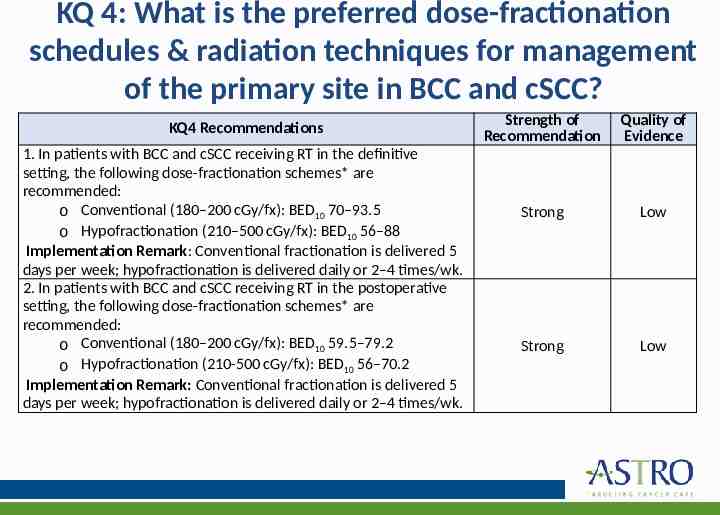
KQ 4: What is the preferred dose-fractionation schedules & radiation techniques for management of the primary site in BCC and cSCC? KQ4 Recommendations 1. In patients with BCC and cSCC receiving RT in the definitive setting, the following dose-fractionation schemes* are recommended: o Conventional (180–200 cGy/fx): BED10 70–93.5 o Hypofractionation (210–500 cGy/fx): BED10 56–88 Implementation Remark: Conventional fractionation is delivered 5 days per week; hypofractionation is delivered daily or 2–4 times/wk. 2. In patients with BCC and cSCC receiving RT in the postoperative setting, the following dose-fractionation schemes* are recommended: o Conventional (180–200 cGy/fx): BED10 59.5–79.2 o Hypofractionation (210-500 cGy/fx): BED10 56–70.2 Implementation Remark: Conventional fractionation is delivered 5 days per week; hypofractionation is delivered daily or 2–4 times/wk. Strength of Recommendation Quality of Evidence Strong Low Strong Low
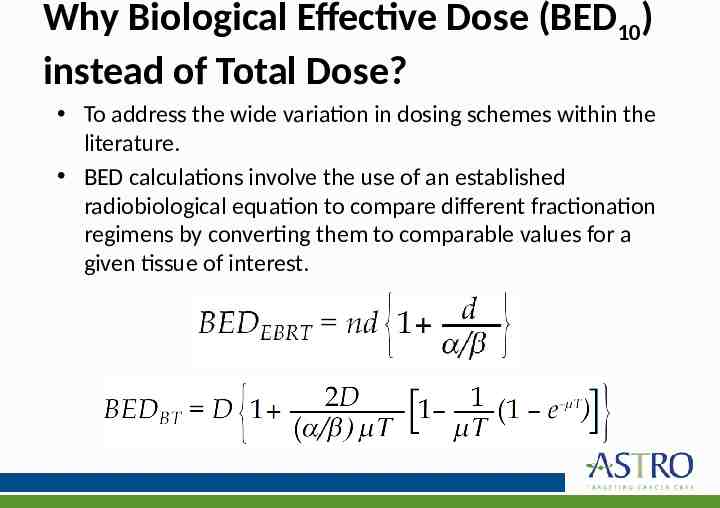
Why Biological Effective Dose (BED10) instead of Total Dose? To address the wide variation in dosing schemes within the literature. BED calculations involve the use of an established radiobiological equation to compare different fractionation regimens bybeam converting them comparable values for a External (EBRT) &tobrachytherapy (BT) given tissue of interest. low-dose rate with significant repair of damage during delivery
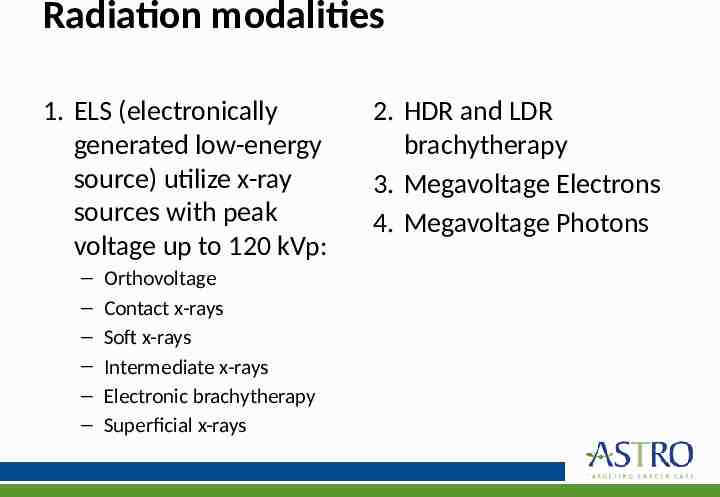
Radiation modalities 1. ELS (electronically generated low-energy source) utilize x-ray sources with peak voltage up to 120 kVp: – – – – – – Orthovoltage Contact x-rays Soft x-rays Intermediate x-rays Electronic brachytherapy Superficial x-rays 2. HDR and LDR brachytherapy 3. Megavoltage Electrons 4. Megavoltage Photons

Local control with varying radiation modalities 5-year local control for ELS, brachytherapy and electrons: 75%-100% 5-year local control for photon therapy: 54%-80% No long-term studies ( 10 years) for electronic brachytherapy in regards to local control and toxicity
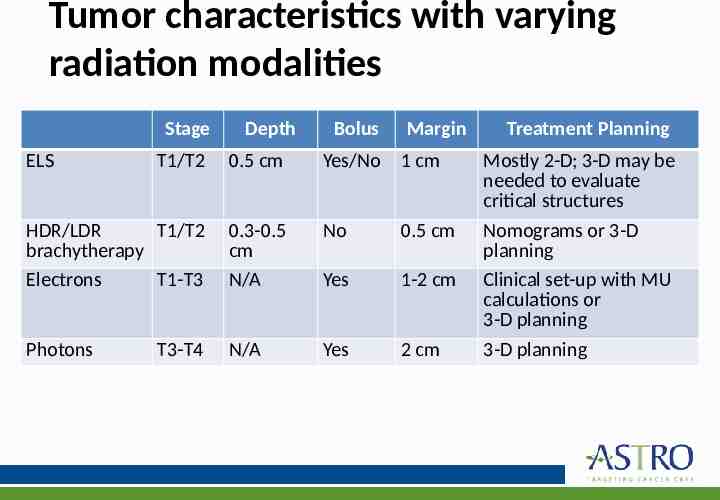
Tumor characteristics with varying radiation modalities Stage ELS Depth Bolus Margin Treatment Planning T1/T2 0.5 cm Yes/No 1 cm Mostly 2-D; 3-D may be needed to evaluate critical structures HDR/LDR T1/T2 brachytherapy Electrons T1-T3 0.3-0.5 cm N/A No 0.5 cm Yes 1-2 cm Nomograms or 3-D planning Clinical set-up with MU calculations or 3-D planning Photons N/A Yes 2 cm 3-D planning T3-T4
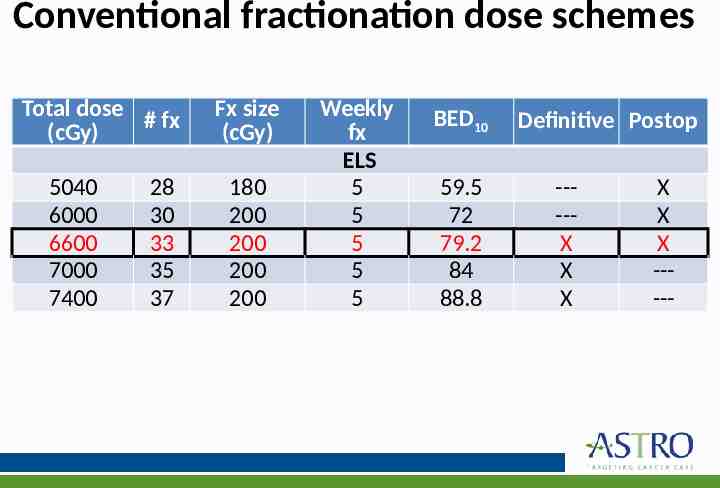
Conventional fractionation dose schemes Total dose # fx (cGy) 5040 6000 6600 7000 7400 28 30 33 35 37 Fx size (cGy) 180 200 200 200 200 Weekly fx ELS 5 5 5 5 5 BED10 59.5 72 79.2 84 88.8 Definitive Postop ----X X X X X X -----
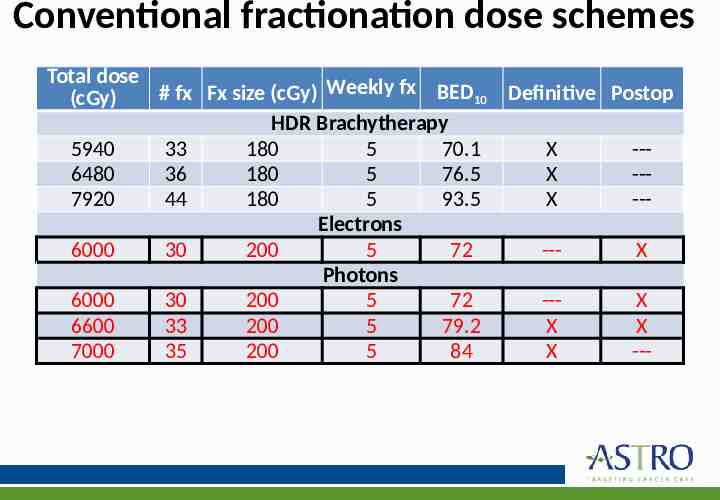
Conventional fractionation dose schemes Total dose # fx Fx size (cGy) Weekly fx BED10 Definitive Postop (cGy) HDR Brachytherapy 5940 33 180 5 70.1 X --6480 36 180 5 76.5 X --7920 44 180 5 93.5 X --Electrons 6000 30 200 5 72 --X Photons 6000 30 200 5 72 --X 6600 33 200 5 79.2 X X 7000 35 200 5 84 X ---
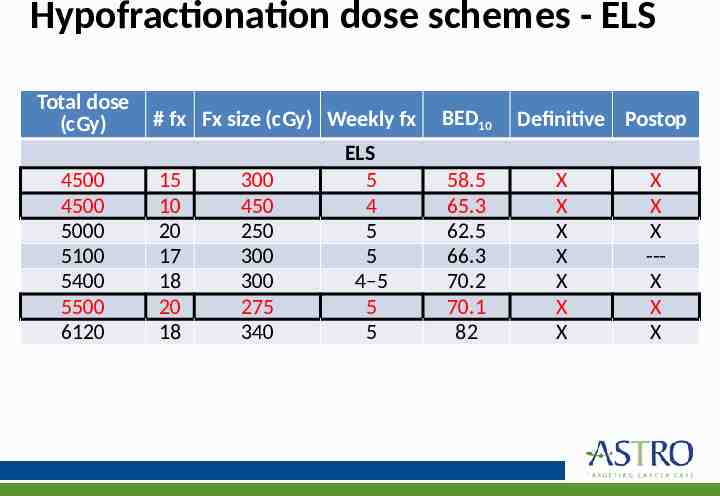
Hypofractionation dose schemes - ELS Total dose (cGy) 4500 4500 5000 5100 5400 5500 6120 # fx Fx size (cGy) Weekly fx 15 10 20 17 18 20 18 300 450 250 300 300 275 340 ELS 5 4 5 5 4–5 5 5 BED10 58.5 65.3 62.5 66.3 70.2 70.1 82 Definitive Postop X X X X X X X X X X --X X X
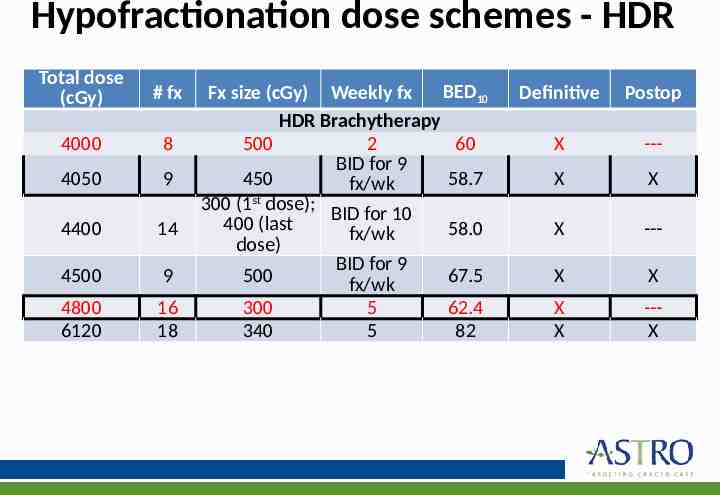
Hypofractionation dose schemes - HDR Total dose (cGy) # fx 4000 8 4050 9 4400 14 4500 9 4800 6120 16 18 BED10 Fx size (cGy) Weekly fx HDR Brachytherapy 500 2 60 BID for 9 450 58.7 fx/wk 300 (1st dose); BID for 10 400 (last 58.0 fx/wk dose) BID for 9 500 67.5 fx/wk 300 5 62.4 340 5 82 Definitive Postop X --- X X X --- X X X X --X
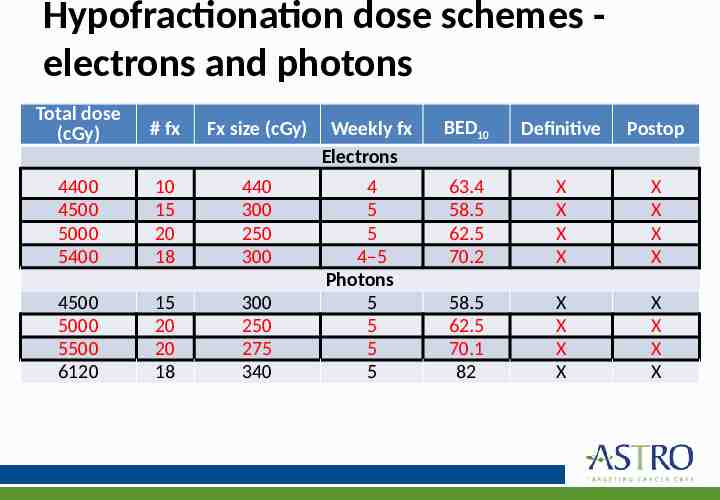
Hypofractionation dose schemes electrons and photons Total dose (cGy) # fx Fx size (cGy) 4400 4500 5000 5400 10 15 20 18 440 300 250 300 4500 5000 5500 6120 15 20 20 18 300 250 275 340 Weekly fx Electrons BED10 Definitive Postop 4 5 5 4–5 Photons 5 5 5 5 63.4 58.5 62.5 70.2 X X X X X X X X 58.5 62.5 70.1 82 X X X X X X X X
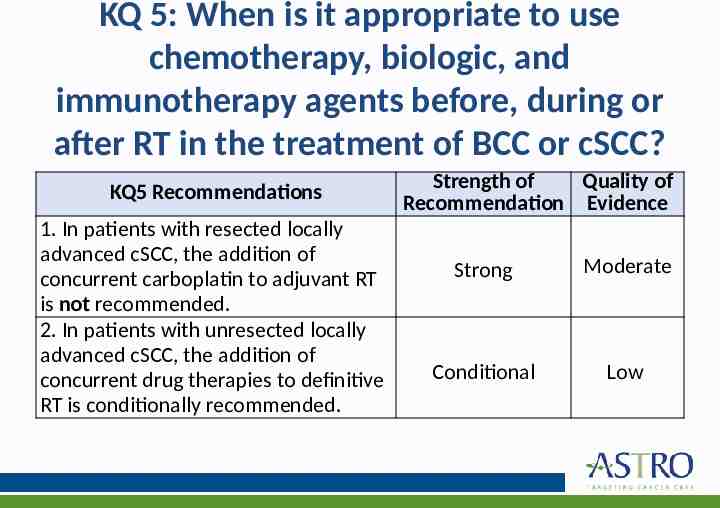
KQ 5: When is it appropriate to use chemotherapy, biologic, and immunotherapy agents before, during or after RT in the treatment of BCC or cSCC? KQ5 Recommendations 1. In patients with resected locally advanced cSCC, the addition of concurrent carboplatin to adjuvant RT is not recommended. 2. In patients with unresected locally advanced cSCC, the addition of concurrent drug therapies to definitive RT is conditionally recommended. Strength of Quality of Recommendation Evidence Strong Moderate Conditional Low
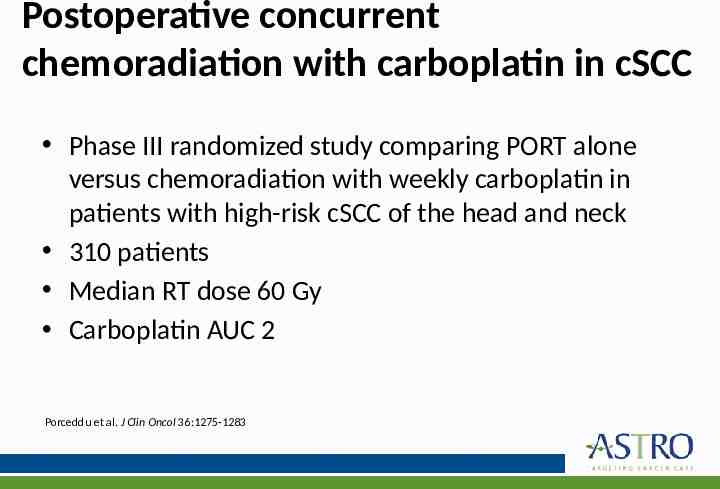
Postoperative concurrent chemoradiation with carboplatin in cSCC Phase III randomized study comparing PORT alone versus chemoradiation with weekly carboplatin in patients with high-risk cSCC of the head and neck 310 patients Median RT dose 60 Gy Carboplatin AUC 2 Porceddu et al. J Clin Oncol 36:1275-1283
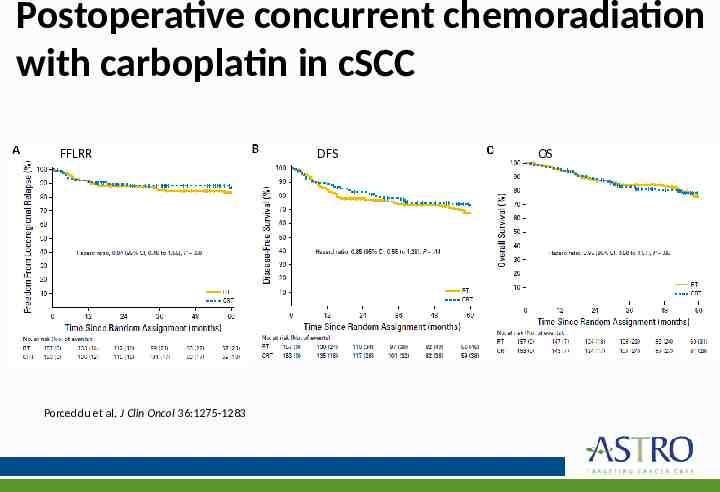
Postoperative concurrent chemoradiation with carboplatin in cSCC FFLRR Porceddu et al. J Clin Oncol 36:1275-1283 DFS OS
Postoperative concurrent chemoradiation with carboplatin in cSCC No significant differences in DFS or OS with the addition of carboplatin to PORT compared with PORT alone 2- and 5-year freedom from locoregional relapse rates were 88% and 83% for RT vs 89% and 87% for chemoradiation No observed enhancement of RT toxicity with carboplatin Porceddu et al. J Clin Oncol 36:1275-1283

Key take away messages Definitive RT for BCC and cSCC is associated with high rates of disease control High-risk features for recurrence after surgery may warrant PORT to the primary bed or regional nodal basins Several dose and fractionation schemes for treatment of BCC and cSCC are preferred Concurrent carboplatin with adjuvant RT is not recommended






