Counting Atoms Balancing Equations Law of Conservation of Mass
10 Slides67.03 KB

Counting Atoms Balancing Equations Law of Conservation of Mass
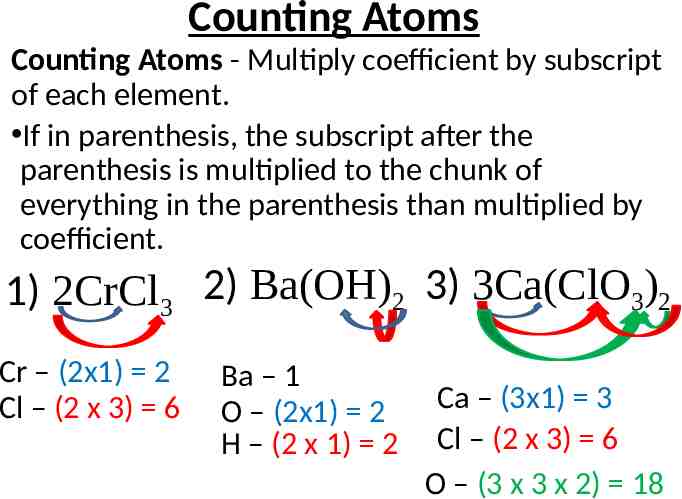
Counting Atoms Counting Atoms - Multiply coefficient by subscript of each element. If in parenthesis, the subscript after the parenthesis is multiplied to the chunk of everything in the parenthesis than multiplied by coefficient. 1) 2CrCl3 2) Ba(OH)2 3) 3Ca(ClO3)2 Cr – (2x1) 2 Cl – (2 x 3) 6 Ba – 1 O – (2x1) 2 H – (2 x 1) 2 Ca – (3x1) 3 Cl – (2 x 3) 6 O – (3 x 3 x 2) 18

Balancing chemical equations reactants 1. Every atom in becomes part of the products . 2. Atoms are never or created destroyed 3. Scientists know that there must be the same number and type of atoms on each of the equation. side 3. Balance by using coefficients in front of the chemical formulas because you can not add or change of a correct subscripts formula

Steps to Balancing Equations 1) Determine the number of atoms for each element. 2) Pick an element that is not equal on both sides of the equation. 3) Add a coefficient in front of the formula with that element and adjust your counts. 4) Continue adding coefficients to get the same number of atoms of each element on both sides
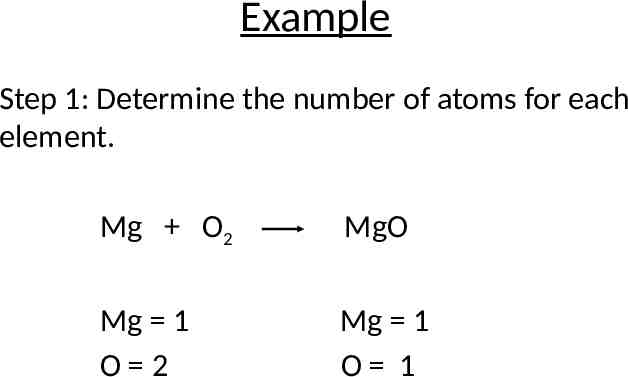
Example Step 1: Determine the number of atoms for each element. Mg O2 MgO Mg 1 O 2 Mg 1 O 1
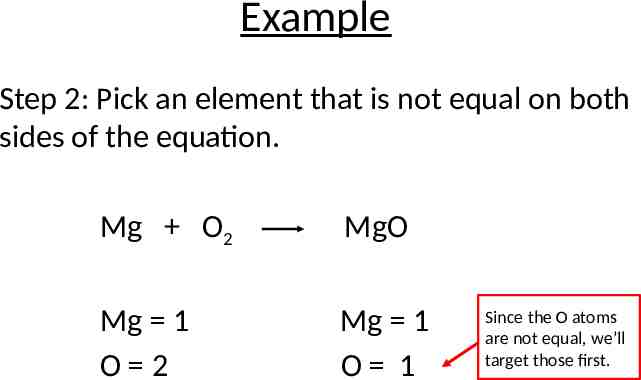
Example Step 2: Pick an element that is not equal on both sides of the equation. Mg O2 MgO Mg 1 O 2 Mg 1 O 1 Since the O atoms are not equal, we’ll target those first.
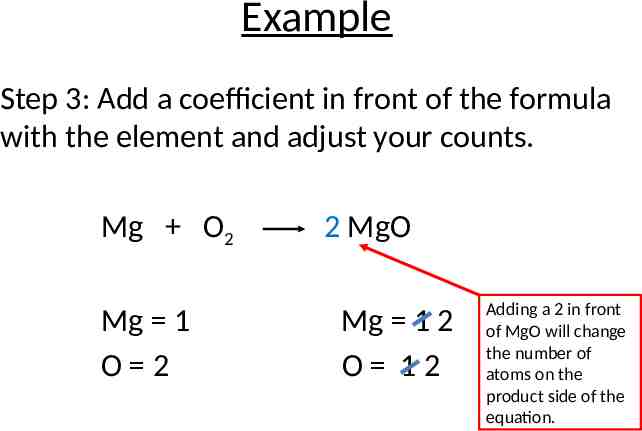
Example Step 3: Add a coefficient in front of the formula with the element and adjust your counts. Mg O2 Mg 1 O 2 2 MgO Mg 1 2 O 12 Adding a 2 in front of MgO will change the number of atoms on the product side of the equation.
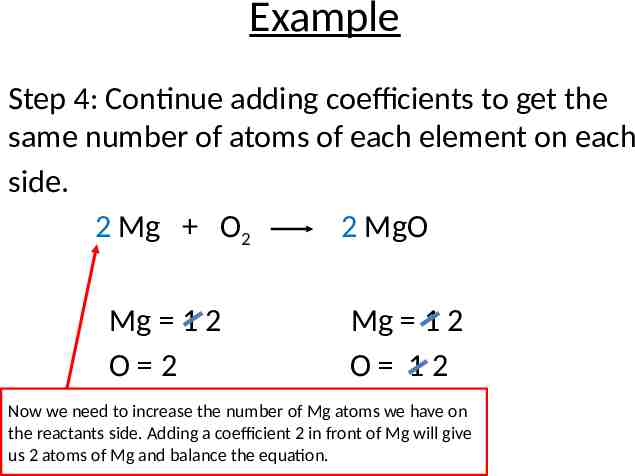
Example Step 4: Continue adding coefficients to get the same number of atoms of each element on each side. 2 Mg O2 2 MgO Mg 1 2 O 2 Mg 1 2 O 12 Now we need to increase the number of Mg atoms we have on the reactants side. Adding a coefficient 2 in front of Mg will give us 2 atoms of Mg and balance the equation.

LAW OF CONSERVATION OF MASS Law of Conservation of mass – MASS CANNOT BE CREATED OR DESTROYED DURING A CHEMICAL REACTION. The mass of all the REACTANTS combined must equal the mass of all the PRODUCTS combined 1. Antoine Lavoisier came up with this law

How does a balanced equation illustrate the Law of Conservation of Mass A balanced chemical equation shows the same number and kind of atoms in both the reactants and the products. Therefore, the total mass of all the atoms in the reactants is the same as the total mass of the same atoms in the products.






