Best Practices for OINDP Pharmaceutical Development Programs
35 Slides461.50 KB

Best Practices for OINDP Pharmaceutical Development Programs Leachables and Extractables III. Safety Evaluation of Extractables and Leachables PQRI Leachables & Extractables Working Group Douglas J. Ball, MS, DABT PQRI Training Course September 20-21, 2006 Washington, DC

L&E Hypothesis Scientifically justifiable thresholds can be developed for the reporting and safety qualification of leachables in OINDP and the reporting of extractables from critical components used in corresponding container/closure systems. Safety qualification of extractables would be scientifically justified on a case-by- case basis

Definitions – Safety Concern & Qualification Thresholds Safety Concern Threshold: Dose below which concern for carcinogenicity and noncarcinogenic toxicity is negligible Identification of leachables below this threshold generally would not be necessary Qualification Threshold Dose below which concern for noncarcinogenic toxicity is negligible Leachables below this threshold without structural alerts for carcinogenicity or irritation would not require compound-specific risk assessment
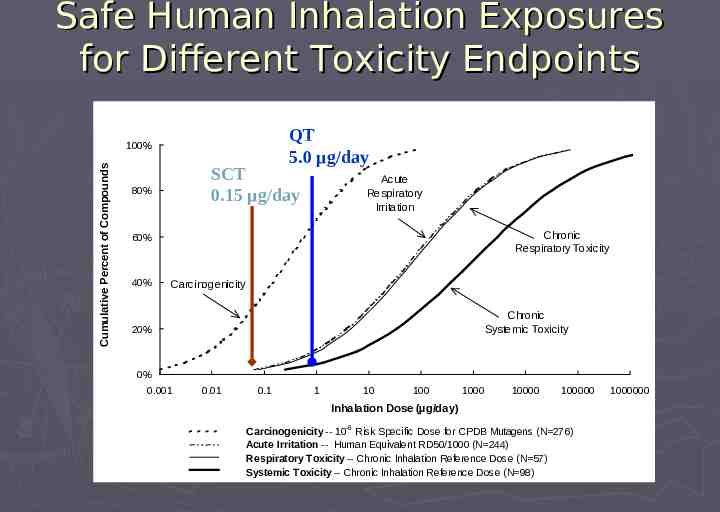
Safe Human Inhalation Exposures for Different Toxicity Endpoints QT 5.0 µg/day Cumulative Percent of Compounds 100% SCT 0.15 µg/day 80% Acute Respiratory Irritation Chronic Respiratory Toxicity 60% 40% Carcinogenicity Chronic Systemic Toxicity 20% 0% 0.001 0.01 0.1 1 10 100 1000 10000 100000 Inhalation Dose (µg/day) Carcinogenicity -- 10-6 Risk Specific Dose for CPDB Mutagens (N 276) Acute Irritation -- Human Equivalent RD50/1000 (N 244) Respiratory Toxicity -- Chronic Inhalation Reference Dose (N 57) Systemic Toxicity -- Chronic Inhalation Reference Dose (N 98) 1000000

Safety Concern Threshold (SCT) is Based on Carcinogenicity Risk Carcinogenicity typically occurs at lower intakes than noncarcinogenic toxicity Thus, intakes with acceptable cancer-risk entail negligible concern for noncarcinogenic toxicity Based on quantitative risk estimates, the SCT limits carcinogenicity risk of unidentified leachables to an acceptable level (10-6) Similar to approach for FDA Threshold of Regulation for indirect food additives, but with some methodological differences

Carcinogenicity Risk Approaches and Assumptions to Derive Threshold Based on distribution of 10-6 risk-specific doses Extrapolated from TD50 values in Carcinogen Potency Database (CPDB) For genotoxic (SAL-positive) carcinogens Assumes potency via inhalation comparable to other routes (principally oral) Extrapolation used: Allometric dose-scaling Central risk estimates rather than upper bound Geometric mean rather than most sensitive species

Based on Genotoxic Carcinogens Genotoxic (SAL-positive) carcinogens are particularly relevant for safety concern: More potent than SAL-negative carcinogens Linear extrapolation to zero risk (ie, no risk-free dose) more applicable to genotoxic carcinogens Most known human carcinogens are genotoxic Structural alerts are more predictive for genotoxins
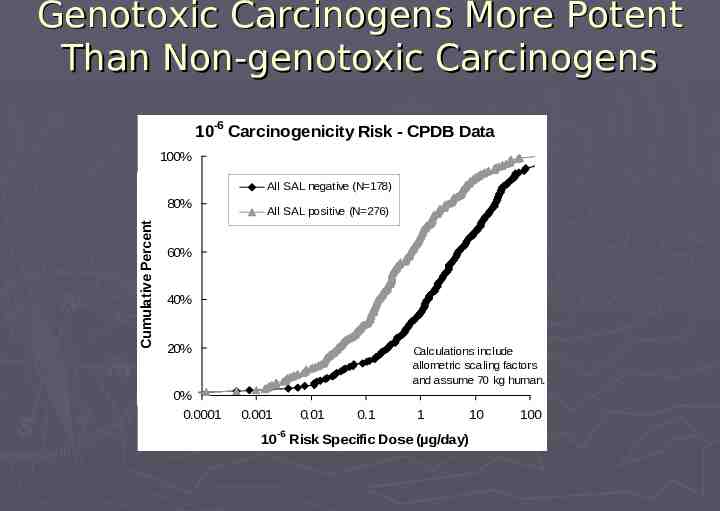
Genotoxic Carcinogens More Potent Than Non-genotoxic Carcinogens 10-6 Carcinogenicity Risk - CPDB Data 100% Cumulative Percent Cumulative Percent All SAL negative (N 178) 80% All SAL positive (N 276) 60% 40% 20% 0% 0.0001 Calculations include allometric scaling factors and assume 70 kg human. 0.001 0.01 -6 0.1 1 10 10 Risk Specific Dose (µg/day) 100
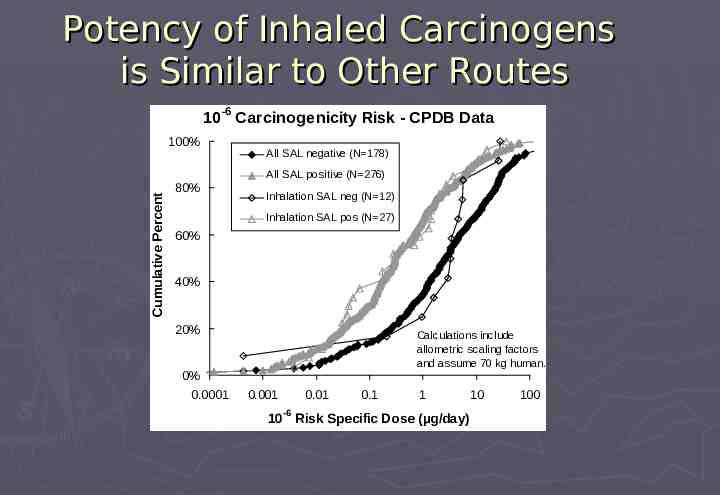
Potency of Inhaled Carcinogens is Similar to Other Routes -6 10 Carcinogenicity Risk - CPDB Data 100% All SAL negative (N 178) Cumulative Percent All SAL positive (N 276) 80% Inhalation SAL neg (N 12) Inhalation SAL pos (N 27) 60% 40% 20% 0% 0.0001 Calculations include allometric scaling factors and assume 70 kg human. 0.001 0.01 -6 0.1 1 10 10 Risk Specific Dose (µg/day) 100
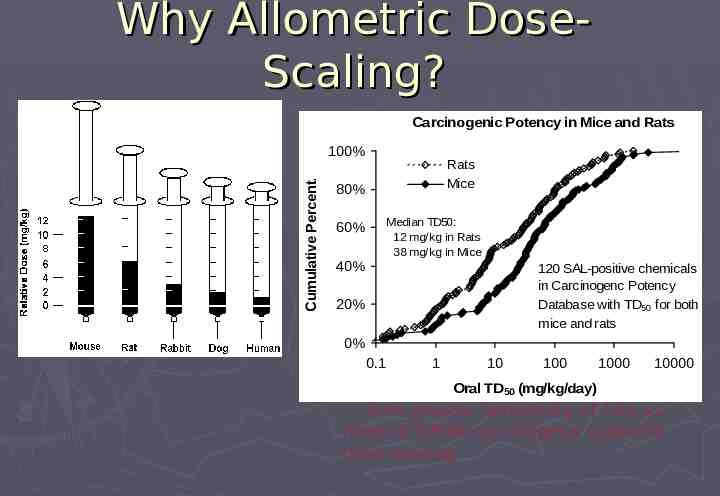
Why Allometric DoseScaling? Carcinogenic Potency in Mice and Rats Cumulative Percent. 100% Rats Mice 80% Median TD50: 12 mg/kg in Rats 38 mg/kg in Mice 60% 40% 120 SAL-positive chemicals in Carcinogenc Potency Database with TD50 for both mice and rats 20% 0% 0.1 1 10 100 1000 10000 Oral TD50 (mg/kg/day) 3-4x greater sensitivity of rats vs mice to CPDB carcinogens supports dose-scaling

Safety Concern Threshold Corresponds to the 37th percentile of SAL-positive carcinogens in the CPDB Median excess cancer risk for a SAL-positive carcinogen at 0.15 µg/day is 0. 41 x 10-6 If 20% of random chemicals are genotoxic carcinogens, 7% of all compounds would exceed 10-6 increased cancer risk at intakes 0.15 µg/day lifetime exposure

Safety Concern Threshold Unknown leachables in OINDP at intakes below a Safety Concern Threshold of 0.15 µg/day present negligible concern for carcinogenic or non-carcinogenic health risks Identification of leachables below this threshold is generally not necessary Exception: some specific, highly potent leachables (eg, nitrosamines, PAHs) may need identification at lower levels

Qualification Threshold is Based on Non-Carcinogenic Toxicity Endpoints Chronic Respiratory & Systemic Toxicity Distribution of chronic “Reference Exposures” US EPA – Inhalation Reference Dose (RfD) CAL EPA – Reference Exposure Level (REL) ATSDR – Minimal Risk Level (MRL) Exposures presenting negligible human risk for noncarcinogenic toxicity; typically based on animal NOAEL and safety factor ( 100x) Acute Irritation Potential Distribution of acute exposure limits NIOSH – Short Term Exposure Limit (STEL) CAL Acute REL RD50/1000 (added safety factor for asthmatics)
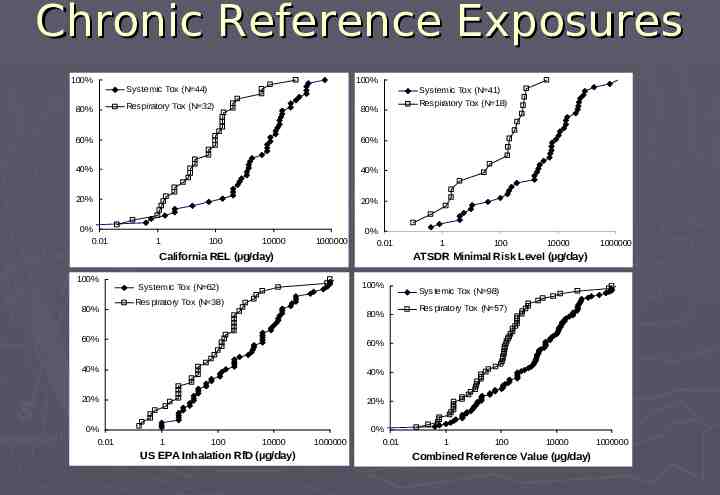
Chronic Reference Exposures 100% 80% 100% Systemic Tox (N 44) Systemic Tox (N 41) Respiratory Tox (N 32) 80% 60% 60% 40% 40% 20% 20% 0% 0.01 1 100 10000 1000000 0% 0.01 California REL (µg/day) 100% 80% 100% Systemic Tox (N 62) Respiratory Tox (N 38) 80% 60% 40% 40% 20% 20% 1 100 10000 US EPA Inhalation RfD (µg/day) 1 100 10000 1000000 ATSDR Minimal Risk Level (µg/day) 60% 0% 0.01 Respiratory Tox (N 18) 1000000 0% 0.01 Systemic Tox (N 98) Respiratory Tox (N 57) 1 100 10000 Combined Reference Value (µg/day) 1000000

Acute Respiratory Irritation Evaluated from point of view that asthmatics are the most sensitive population Used mouse RD50 database as starting point Validated, well-accepted, extensive database of commodity chemicals RD50 is the inhaled concentration that reduces respiratory frequency by 50% Correlation of RD50 to human exposure RD50 x 0.1 tolerable acute human exposure RD50 x 0.03 daily exposure limit (TLV-TWA) RD50 x 0.001 x 10 minutes should be safe for most asthmatics
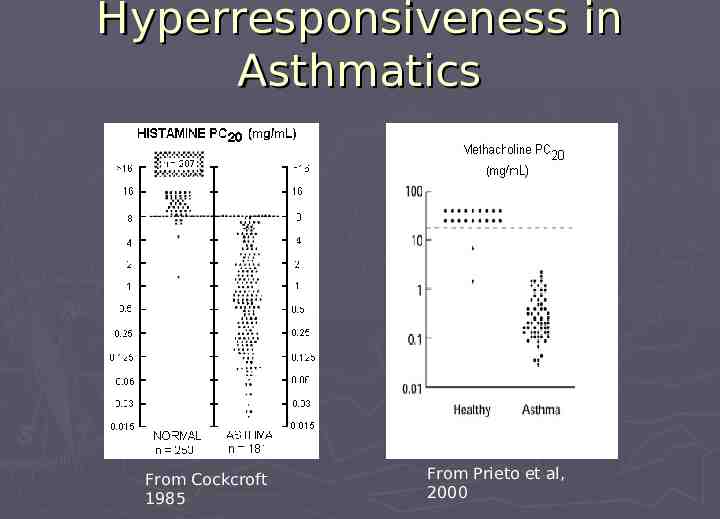
Hyperresponsiveness in Asthmatics From Cockcroft 1985 From Prieto et al, 2000
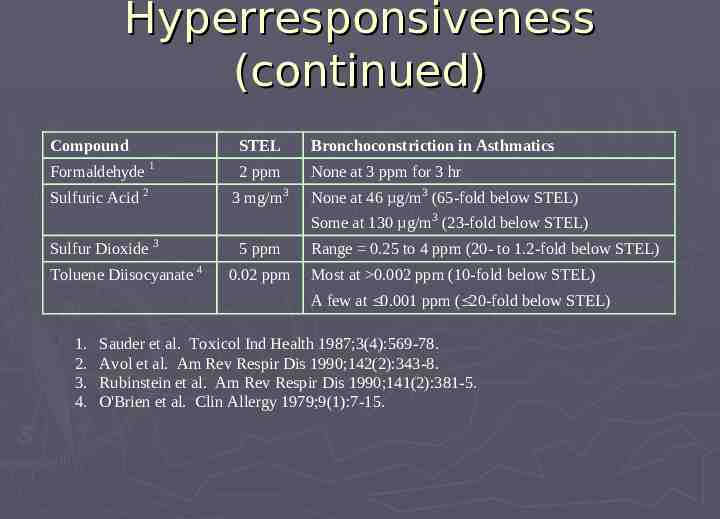
Hyperresponsiveness (continued) Compound STEL Bronchoconstriction in Asthmatics Formaldehyde 1 2 ppm None at 3 ppm for 3 hr Sulfuric Acid 2 3 mg/m3 None at 46 µg/m3 (65-fold below STEL) Some at 130 µg/m3 (23-fold below STEL) Sulfur Dioxide 3 Toluene Diisocyanate 4 5 ppm 0.02 ppm Range 0.25 to 4 ppm (20- to 1.2-fold below STEL) Most at 0.002 ppm (10-fold below STEL) A few at 0.001 ppm ( 20-fold below STEL) 1. 2. 3. 4. Sauder et al. Toxicol Ind Health 1987;3(4):569-78. Avol et al. Am Rev Respir Dis 1990;142(2):343-8. Rubinstein et al. Am Rev Respir Dis 1990;141(2):381-5. O'Brien et al. Clin Allergy 1979;9(1):7-15.
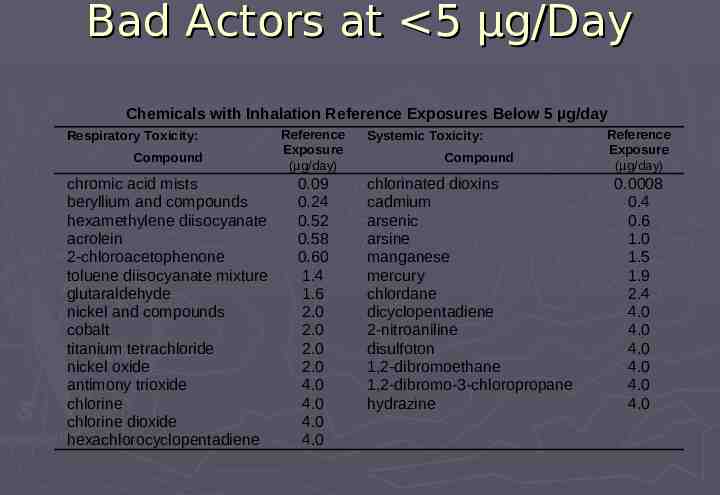
Bad Actors at 5 µg/Day Chemicals with Inhalation Reference Exposures Below 5 µg/day Respiratory Toxicity: Compound chromic acid mists beryllium and compounds hexamethylene diisocyanate acrolein 2-chloroacetophenone toluene diisocyanate mixture glutaraldehyde nickel and compounds cobalt titanium tetrachloride nickel oxide antimony trioxide chlorine chlorine dioxide hexachlorocyclopentadiene Reference Exposure (µg/day) 0.09 0.24 0.52 0.58 0.60 1.4 1.6 2.0 2.0 2.0 2.0 4.0 4.0 4.0 4.0 Systemic Toxicity: Compound chlorinated dioxins cadmium arsenic arsine manganese mercury chlordane dicyclopentadiene 2-nitroaniline disulfoton 1,2-dibromoethane 1,2-dibromo-3-chloropropane hydrazine Reference Exposure (µg/day) 0.0008 0.4 0.6 1.0 1.5 1.9 2.4 4.0 4.0 4.0 4.0 4.0 4.0

Qualification Threshold Corresponds to: 9th percentile of Reference Exposures for systemic toxicity 21st percentile of Reference Exposures for respiratory toxicity 22nd percentile of RD50/1000 Incorporates large safety margins for chronic toxicity Most chemicals of concern below 5 µg/day have obvious structural alerts for irritation or toxicity (eg, heavy metals, aldehydes, isocyanates, organic phosphates) Leachables below this threshold without structural alerts for carcinogenicity or irritation should not require compound-specific risk assessment

Integrated Approach to Developing a Container Closure System

Integrated Approach (1) Materials purchased from supplier Container Closure Team (CCT) formed Comprised of chemists, toxicologists, packaging, procurement Quality materials chosen by the CCT Suppliers provide formulation and general extract information Cost a secondary factor Toxicologist evaluates material extract profile Initial evaluation made early in the container closure development process Safety information from the supplier (e.g., USP results) In silico evaluation Preliminary qualification of each material based on accepted safety thresholds and anticipated TDI

Integrated Approach (2) Container Closure developed for DP Total extract profiling conducted by chemists Analytical methods based on material formulation and general extract profile obtained from supplier Analytical LOD based on qualification thresholds Toxicological Qualification of CCS Based on leachable profile of DP Leachables below SCT considered qualified Leachables greater than the SCT and less than the QT Leachables above the QT can be qualified on a case-by-case basis Qualify with in silico data and published data Additional in vitro, in vivo testing on a case-by-case basis Develop qualification strategy and work with regulators to establish qualification of leachables above the QT Acceptable risk assessment of leachable profile No registration delay due to safety issues related to CCS
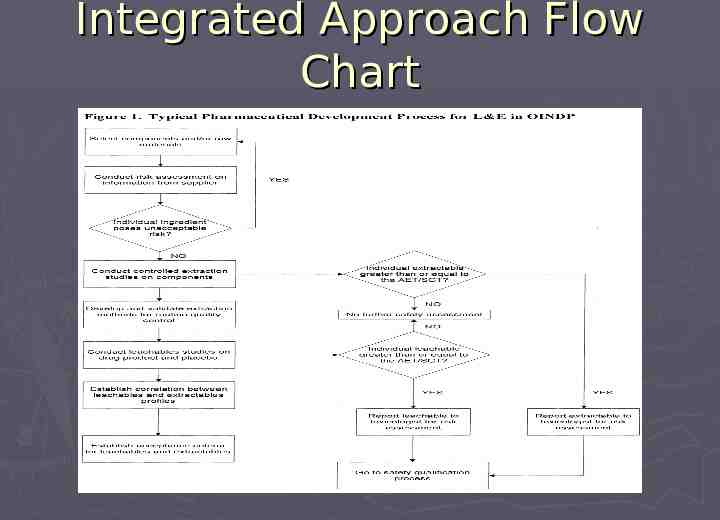
Integrated Approach Flow Chart
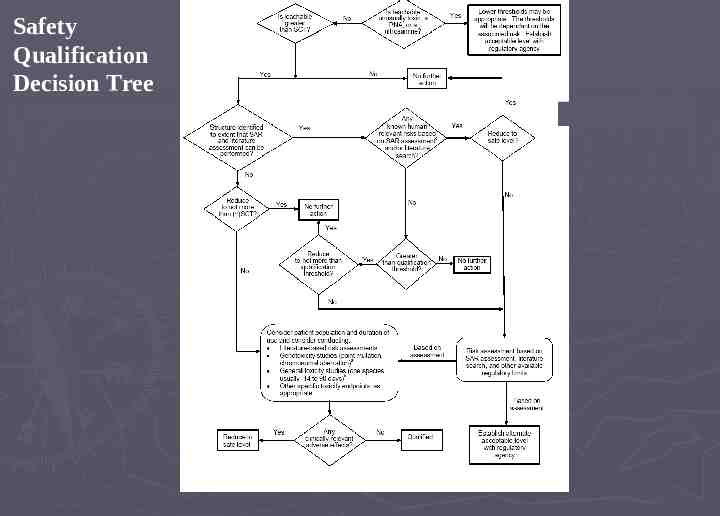
Safety Qualification Decision Tree
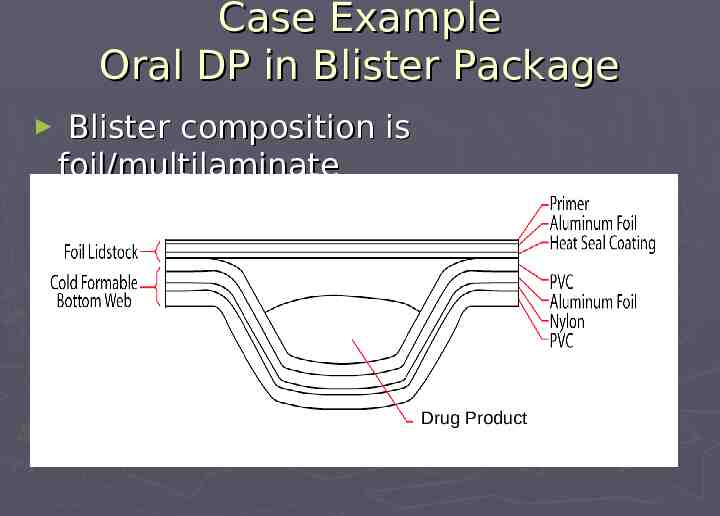
Case Example Oral DP in Blister Package Blister composition is foil/multilaminate Drug Product
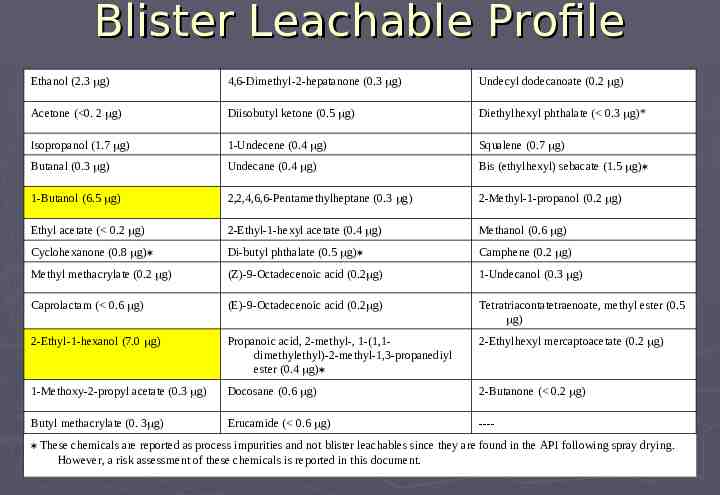
Blister Leachable Profile Ethanol (2.3 g) 4,6-Dimethyl-2-hepatanone (0.3 g) Undecyl dodecanoate (0.2 g) Acetone ( 0. 2 g) Diisobutyl ketone (0.5 g) Diethylhexyl phthalate ( 0.3 g)* Isopropanol (1.7 g) 1-Undecene (0.4 g) Squalene (0.7 g) Butanal (0.3 g) Undecane (0.4 g) Bis (ethylhexyl) sebacate (1.5 g) 1-Butanol (6.5 g) 2,2,4,6,6-Pentamethylheptane (0.3 g) 2-Methyl-1-propanol (0.2 g) Ethyl acetate ( 0.2 g) 2-Ethyl-1-hexyl acetate (0.4 g) Methanol (0.6 g) Cyclohexanone (0.8 g) Di-butyl phthalate (0.5 g) Camphene (0.2 g) Methyl methacrylate (0.2 g) (Z)-9-Octadecenoic acid (0.2 g) 1-Undecanol (0.3 g) Caprolactam ( 0.6 g) (E)-9-Octadecenoic acid (0.2 g) Tetratriacontatetraenoate, methyl ester (0.5 g) 2-Ethyl-1-hexanol (7.0 g) Propanoic acid, 2-methyl-, 1-(1,1dimethylethyl)-2-methyl-1,3-propanediyl ester (0.4 g) 2-Ethylhexyl mercaptoacetate (0.2 g) 1-Methoxy-2-propyl acetate (0.3 g) Docosane (0.6 g) 2-Butanone ( 0.2 g) Butyl methacrylate (0. 3 g) Erucamide ( 0.6 g) ---- These chemicals are reported as process impurities and not blister leachables since they are found in the API following spray drying. However, a risk assessment of these chemicals is reported in this document.

1-Butanol: Data TDI is 6.5 g, or 0.09 g/kg for a 70 kg person. Negative in the Ames and sister-chromatid exchange assays. Acute oral LD50 values in rats and mice are 790 mg/kg and 2,680 mg/kg, respectively, which classifies it as “moderately toxic.” Acute inhalation (4 hour) LC50 in rats is 8,000 ppm (24.24 mg/L), resulting in a pulmonary dose of 3,989 mg/kg. (24.24 mg/L x 240 min x 0.24 L/min 0.35 kg 3,989 mg/kg) In various oral and inhalation reproductive toxicology studies in rats, several effects have been reported, but the doses that produce them were in the g/kg range. Is permitted as a direct food additive in the United States (21CFR 172.515). In the United States, a reference dose for chronic oral exposure (RfD) for humans of 100 g/kg/day has been established by the Environmental Protection Agency. Thus, this estimates the daily oral intake of 1-butanol that is likely to be without appreciable risk of deleterious effects during a lifetime. In the United States, OSHA has established an 8-hour TWA of 100 ppm (0.303 mg/L) for 1‑butanol. Thus, the total acceptable daily pulmonary dose for a 70 kg person is 18,699 g/kg. Regulatory authorities in several European countries have established the same or a similar TWA. (0.303 mg/L x 480 min x 9 L/min 70 kg 18.699 mg/kg or 18,699 g /kg) It is a Class 3 solvent (ICH Q3C Guideline for Residual Solvents), and therefore it is considered that amounts of 50 mg/day (or 714 g/kg/day for a 70 kg person) would be acceptable without justification.
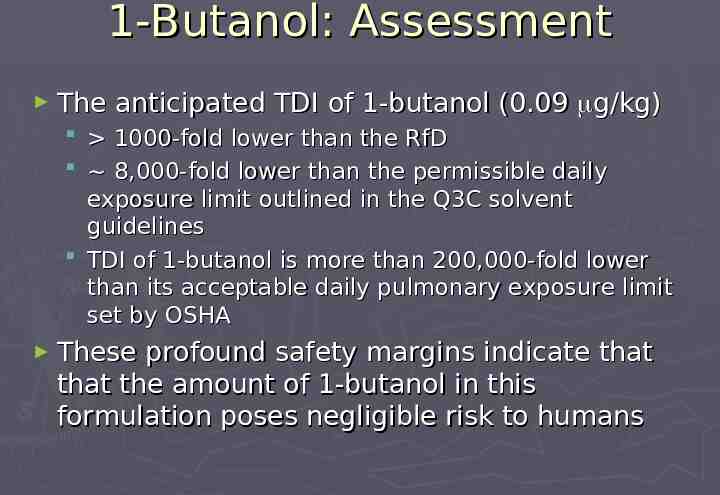
1-Butanol: Assessment The anticipated TDI of 1-butanol (0.09 g/kg) 1000-fold lower than the RfD 8,000-fold lower than the permissible daily exposure limit outlined in the Q3C solvent guidelines TDI of 1-butanol is more than 200,000-fold lower than its acceptable daily pulmonary exposure limit set by OSHA These profound safety margins indicate that that the amount of 1-butanol in this formulation poses negligible risk to humans

1-Butanol: Reference List Center for Drug Evaluation and Research. Guidance for Industry. Q3C Impurities: Residual Solvents (website). Connelly J, Hasegawa R, McArdle J, et al. Residual solvents. Pharmeuropa 1997;9:S54. National Institute of Occupational Safety and Health (website). Ollroge I. Threshold values and recommendations. In: Marquardt H, Schäfer S, McClellan R, et al, editors. Toxicology. New York: Academic Press; 1999: p. 1201-29. Registry of Toxic Effects of Chemical Substances (website). Toxnet (website). Zbinden G. Acute toxicity. In: Zbinden G, editor. Progress in toxicology. New York: Springer-Verlag; 1973: p. 24.

2-Ethyl-1-Hexanol: Data TDI is 7.0 g, or 0.1 g/kg for a 70 kg person. Negative in several genotoxicity assays including the Ames, mammalian cell gene mutation, in vitro cytogenetics, in vivo cytogenetics, UDS (rat hepatocytes), in vitro cell transformation and dominate lethal assay in mice. A weak mutagenic response was reported in the 8‑azaguanine-resistance assay in Salmonella. Characterized as a weak peroxisome proliferator in rats. Acute oral LD50 values in rats and mice are 3,730 mg/kg and 2,500 mg/kg, respectively, which classifies it as “moderately toxic.” Acute inhalation (6 hour) LC50 in rats is 2,000 ppm ( 10.64 mg/L), resulting in a pulmonary dose of 2,627 mg/kg. ( 10.64 mg/L x 360 min x 0.24 L/min 0.35 kg 2,627 mg/kg) In a 3-month inhalation toxicology study in rats, the highest vapor concentration of 120 ppm (0.638 mg/L) administered 6 hours/day was the NOAEL, which resulted in a pulmonary dose of 157 mg/kg/day. (0.638 mg/L x 360 min x 0.24 L/min 0.35 kg 157 mg/kg/day) In a 13-week oral toxicology study in rats and mice, the NOAEL in both species was 125 mg/kg/day. An inhalation teratology study in rats with exposure to a vapor concentration of 0.850 mg/L (7 hours/day) caused no teratogenic effects, which resulted in a pulmonary dose of 245 mg/kg. (0.850 mg/L x 420 min x 0.24 L/min 0.35 kg 245 mg/kg) In an oral reproductive toxicology study in mice, a dose of 191 mg/kg/day caused no developmental findings. In an oral reproductive toxicology study in rats, oral doses ranging from 800-1600 mg/kg/day caused malformations including hydronephrosis, heart malformations, and tail and limb defects. Was not carcinogenic in a rat oral oncogenicity study up to the highest dose of 500 mg/kg/day. The NOAEL for systemic toxicity in this study was 50 mg/kg/day. Was not carcinogenic in a mouse oral oncogenicity study in males up to a highest dose of 750 mg/kg/day, and in females up to 200 mg/kg/day. A weak or equivocal trend in increased incidence of liver tumors occurred in female mice given the highest dose of 750 mg/kg/day. The NOAEL for systemic toxicity in this study was 200 mg/kg/day. No apparent injury has been reported in humans from its use in industry. The probable oral lethal dose in humans is estimated to be from 500 mg/kg - 5,000 mg/kg.

2-Ethyl-1-Hexanol: Assessment TDI of 2-ethyl-1-hexanol (0.1 g/kg) in humans is more than one million-fold lower than doses in animals that failed to produce systemic toxicity (via the pulmonary and oral route) or, carcinogenicity (via the oral route) TDI of 2-ethyl-1-hexanol is at least five million-fold lower than the estimated oral lethal dose in humans Profound safety margin indicates that that the amount of 2-ethyl-1-hexanol in this formulation poses negligible risk to humans

Erucamide: Data TDI is 0.6 g, or 0.009 g/kg for a 70 kg person SAR was negative for genotoxicity and/or carcinogenicity alert (DEREK) The stearyl derivative of erucamide was negative in the Ames test Is permitted as an indirect food additive in the United States (CFR 175.105)

Euracamide: Assessment small dose (i.e., 0.6 µg TDI or 0.009 g/kg for a 70 kg adult) Listed as an indirect food additive No structural alerts for genotoxicity or carcinogenicity Erucamide poses negligible risk to humans

Analytical Evaluation Threshold The Analytical Evaluation Threshold concept converts the SCT (0.15 µg/day for an individual leachable) into a threshold which can be applied to an individual drug product leachables profile, and by extension to a critical component extractables profile. It attempts to address the question: How low do we go?
Leachables Profile – 1 Week Timepoint Expanded Section A bundanc e T IC : 1 1 1 0 0 3 0 3 .D 120000 110000 100000 90000 80000 70000 60000 50000 40000 30000 20000 10000 0 1 7 .8 0 1 8 .0 0 1 8 .2 0 1 8 .4 0 1 8 .6 0 1 8 .8 0 1 9 .0 0 1 9 .2 0 1 9 .4 0 1 9 .6 0 1 9 .8 0 2 0 .0 0 2 0 .2 0 2 0 .4 0 2 0 .6 0 T im e --