Organic Reactions – Regents
14 Slides181.00 KB

Organic Reactions - Regents
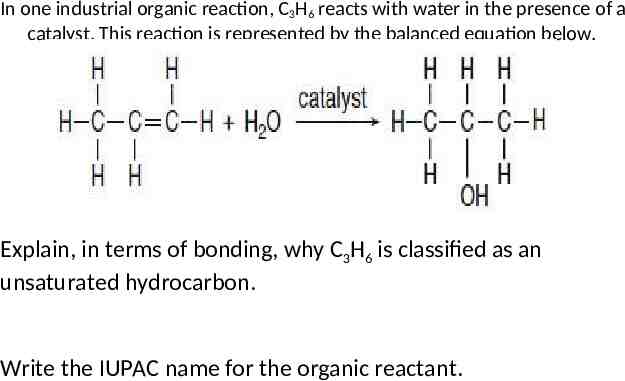
In one industrial organic reaction, C3H6 reacts with water in the presence of a catalyst. This reaction is represented by the balanced equation below. Explain, in terms of bonding, why C3H6 is classified as an unsaturated hydrocarbon. Write the IUPAC name for the organic reactant.
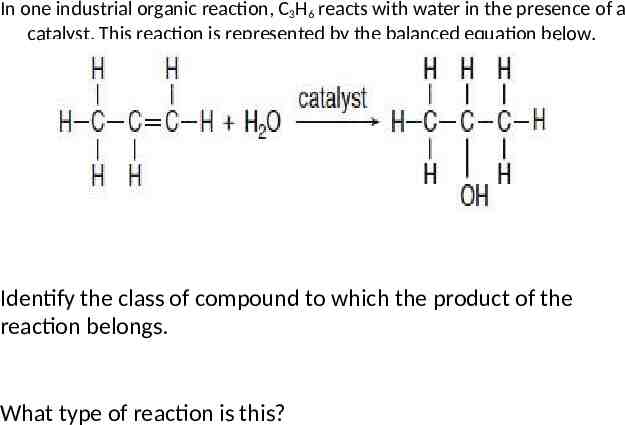
In one industrial organic reaction, C3H6 reacts with water in the presence of a catalyst. This reaction is represented by the balanced equation below. Identify the class of compound to which the product of the reaction belongs. What type of reaction is this?
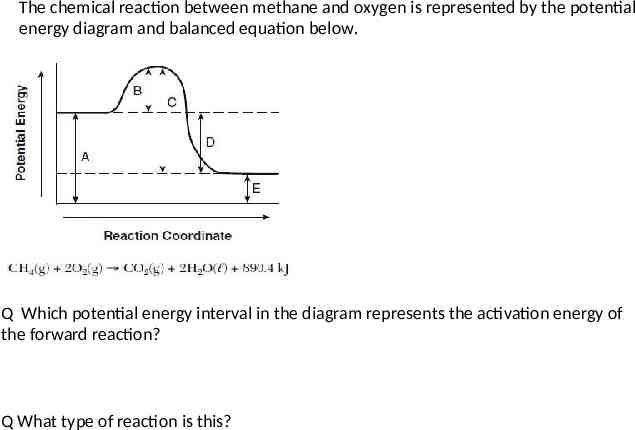
The chemical reaction between methane and oxygen is represented by the potential energy diagram and balanced equation below. Q Which potential energy interval in the diagram represents the activation energy of the forward reaction? Q What type of reaction is this?
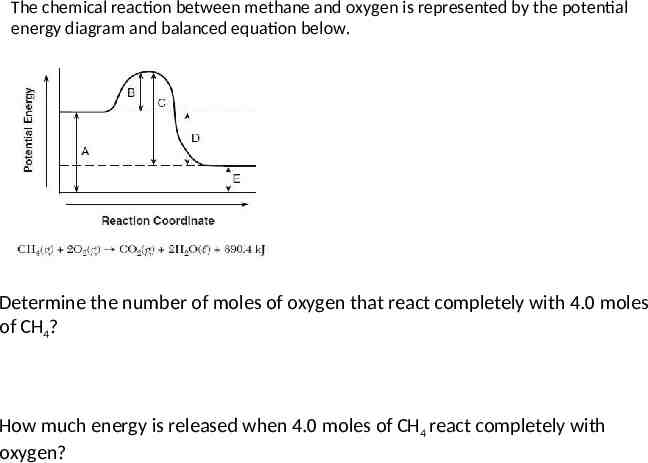
The chemical reaction between methane and oxygen is represented by the potential energy diagram and balanced equation below. Determine the number of moles of oxygen that react completely with 4.0 moles of CH4? How much energy is released when 4.0 moles of CH4 react completely with oxygen?
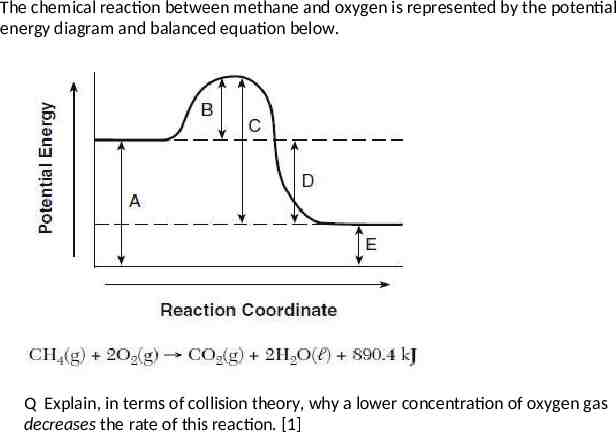
The chemical reaction between methane and oxygen is represented by the potential energy diagram and balanced equation below. Q Explain, in terms of collision theory, why a lower concentration of oxygen gas decreases the rate of this reaction. [1]
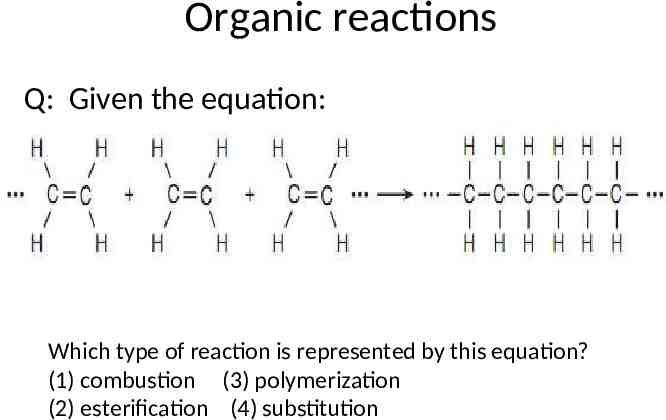
Organic reactions Q: Given the equation: Which type of reaction is represented by this equation? (1) combustion (3) polymerization (2) esterification (4) substitution

Organic reactions Q The reaction between an organic acid and an alcohol produces (1) an aldehyde (3) an ether (2) a ketone (4) an ester
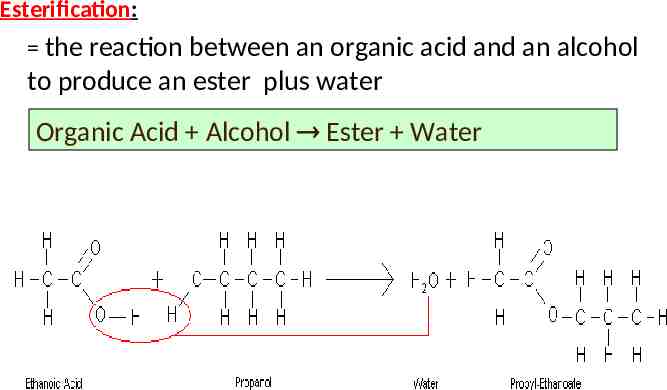
Esterification: the reaction between an organic acid and an alcohol to produce an ester plus water Organic Acid Alcohol Ester Water
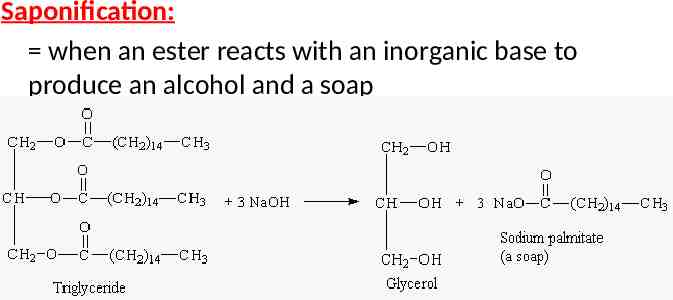
Saponification: when an ester reacts with an inorganic base to produce an alcohol and a soap
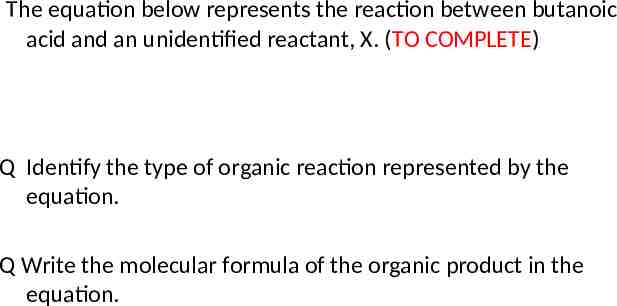
The equation below represents the reaction between butanoic acid and an unidentified reactant, X. (TO COMPLETE) Q Identify the type of organic reaction represented by the equation. Q Write the molecular formula of the organic product in the equation.

The equation below represents the reaction between butanoic acid and an unidentified reactant, X. (TO COMPLETE) Q Draw a structural formula for the unidentified reactant, X, in the equation.
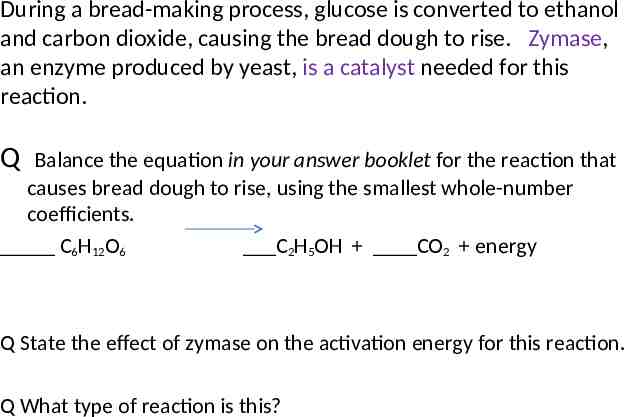
During a bread-making process, glucose is converted to ethanol and carbon dioxide, causing the bread dough to rise. Zymase, an enzyme produced by yeast, is a catalyst needed for this reaction. Q Balance the equation in your answer booklet for the reaction that causes bread dough to rise, using the smallest whole-number coefficients. C6H12O6 C2H5OH CO2 energy Q State the effect of zymase on the activation energy for this reaction. Q What type of reaction is this?

During a bread-making process, glucose is converted to ethanol and carbon dioxide, causing the bread dough to rise. Zymase, an enzyme produced by yeast, is a catalyst needed for this reaction. Q Balance the equation in your answer booklet for the reaction that causes bread dough to rise, using the smallest wholenumber coefficients. C6H12O6 C2H5OH CO2 energy Q In the space in your answer booklet, draw a structural formula for the alcohol formed in this reaction.






