2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for
33 Slides1.25 MB

2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure Developed in Collaboration With the American Academy of Family Physicians, American College of Chest Physicians, and International Society for Heart and Lung Transplantation

Citation This slide set was adapted from the 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure (Journal of the American College of Cardiology). Published on April 28, 2017, available at: Yancy, et. al. ACC/AHA/HFSA 2017 Heart Failure Focused Update The full-text guidelines are also available on the following Web sites: American College of Cardiology (www.acc.org) American Heart Association (professional.heart.org) Heart Failure Society of America(www.hfsa.org)

Special Thanks To The Heart Failure Focused Update Writing Committee Members Clyde W. Yancy, MD, MSc, MACC, FAHA, FHFSA, Chair Mariell Jessup, MD, FACC, FAHA, Vice Chair Biykem Bozkurt, MD, PhD, FACC, FAHA*† Steven M. Hollenberg, MD, FACC# Javed Butler, MD, MBA, MPH, FACC, FAHA*‡ JoAnn Lindenfeld, MD, FACC, FAHA, FHFSA*¶ Donald E. Casey, Jr, MD, MPH, MBA, FACC§ Frederick A. Masoudi, MD, MSPH, FACC** Monica M. Colvin, MD, FAHA Patrick E. McBride, MD, MPH, FACC†† Mark H. Drazner, MD, MSc, FACC, FAHA, FHFSA‡ Pamela N. Peterson, MD, FACC, FAHA‡ Gerasimos S. Filippatos, MD * Lynne Warner Stevenson, MD, FACC*‡ Gregg C. Fonarow, MD, FACC, FAHA, FHFSA*‡ Cheryl Westlake, PhD, RN, ACNS-BC, FAHA , FHFSA, ¶ Michael M. Givertz, MD, FACC, FHFSA*¶ †ACC/AHA Task Force on Clinical Practice Guidelines Liaison. ‡ACC/AHA Representative. §ACP Representative. ISHLT Representative. ¶HFSA Representative. #ACCP Representative. **ACC/AHA Task Force on Performance Measures Representative. ††AAFP Representative. ‡‡Former Task Force member; current member during the writing effort.
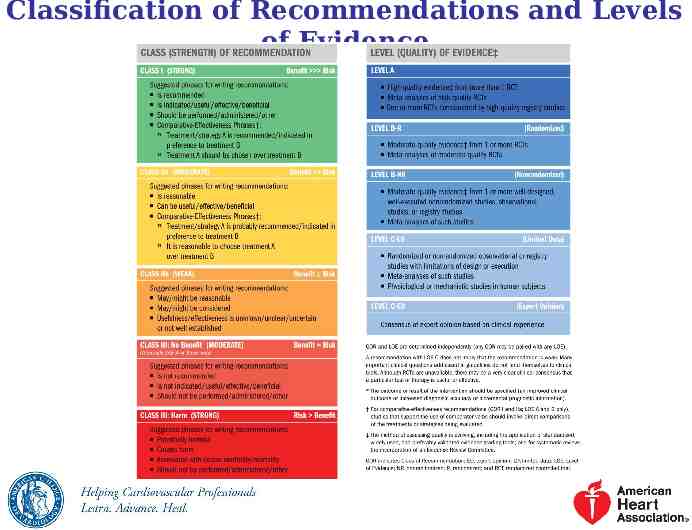
Classification of Recommendations and Levels of Evidence

Introduction The purpose of this focused update is to update the “2013 ACCF/AHA Guideline for the Management of Heart Failure” (2013 HF guideline) in areas where in which new evidence has emerged since its publication. The scope of the focused update includes revision to the sections on – Biomarkers – New therapies indicated for stage C HF with reduced ejection fraction (HFrEF) – Updates on HF with preserved ejection fraction (HFpEF) – New data on important comorbidities, including sleep apnea, anemia, and hypertension – And new insights regarding the prevention of HF

2017 Heart Failure Focused Update Initial and Serial Evaluation of Heart Failure

Initial and Serial Evaluation of Heart Failure Biomarkers
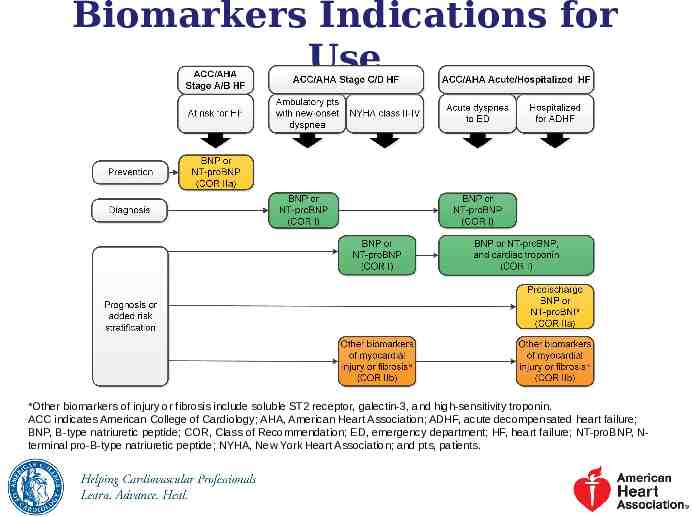
Biomarkers Indications for Use *Other biomarkers of injury or fibrosis include soluble ST2 receptor, galectin-3, and high-sensitivity troponin. ACC indicates American College of Cardiology; AHA, American Heart Association; ADHF, acute decompensated heart failure; BNP, B-type natriuretic peptide; COR, Class of Recommendation; ED, emergency department; HF, heart failure; NT-proBNP, Nterminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; and pts, patients.
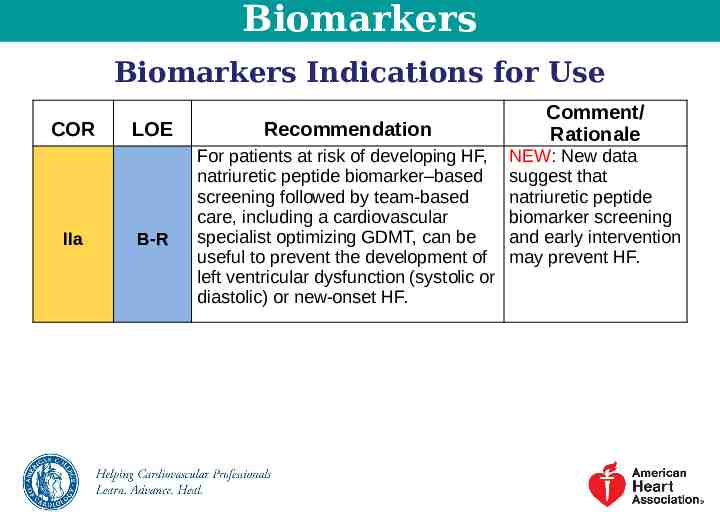
Biomarkers Biomarkers Indications for Use COR IIa LOE B-R Recommendation For patients at risk of developing HF, natriuretic peptide biomarker–based screening followed by team-based care, including a cardiovascular specialist optimizing GDMT, can be useful to prevent the development of left ventricular dysfunction (systolic or diastolic) or new-onset HF. Comment/ Rationale NEW: New data suggest that natriuretic peptide biomarker screening and early intervention may prevent HF.
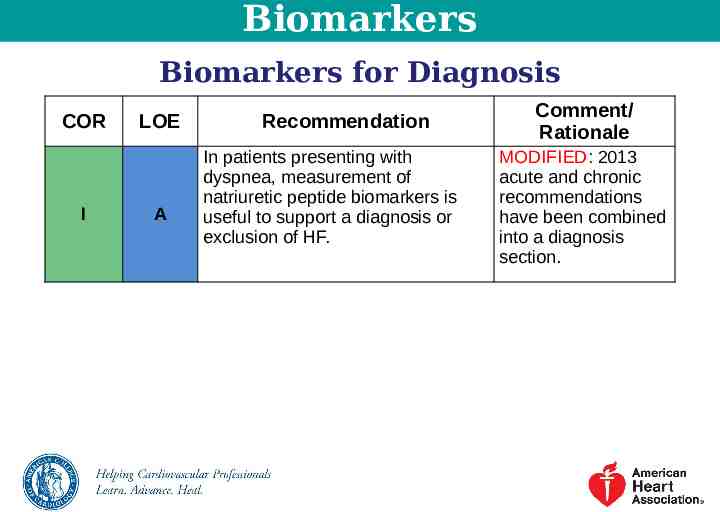
Biomarkers Biomarkers for Diagnosis COR I LOE A Recommendation In patients presenting with dyspnea, measurement of natriuretic peptide biomarkers is useful to support a diagnosis or exclusion of HF. Comment/ Rationale MODIFIED: 2013 acute and chronic recommendations have been combined into a diagnosis section.
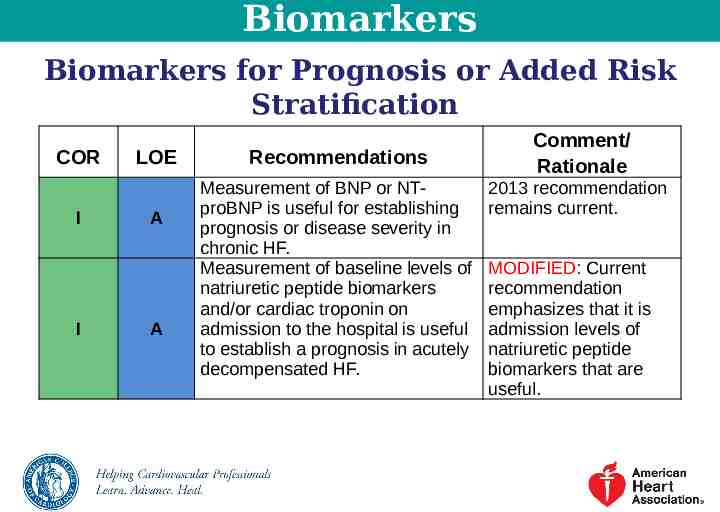
Biomarkers Biomarkers for Prognosis or Added Risk Stratification COR I I LOE A A Recommendations Measurement of BNP or NTproBNP is useful for establishing prognosis or disease severity in chronic HF. Measurement of baseline levels of natriuretic peptide biomarkers and/or cardiac troponin on admission to the hospital is useful to establish a prognosis in acutely decompensated HF. Comment/ Rationale 2013 recommendation remains current. MODIFIED: Current recommendation emphasizes that it is admission levels of natriuretic peptide biomarkers that are useful.
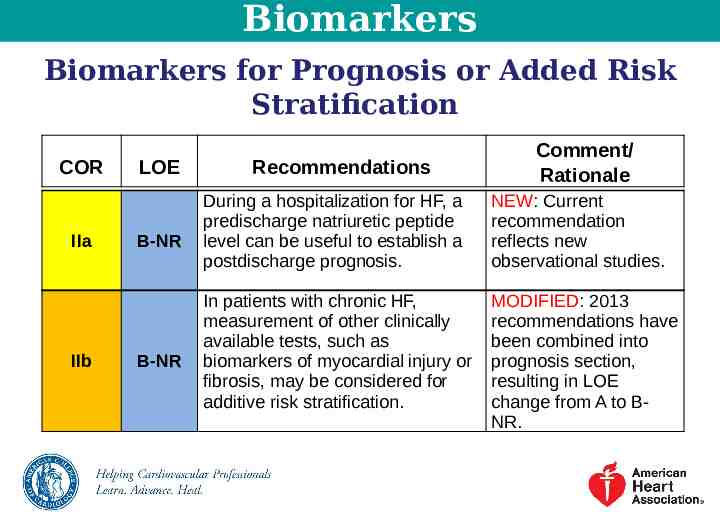
Biomarkers Biomarkers for Prognosis or Added Risk Stratification COR IIa IIb LOE B-NR B-NR Recommendations Comment/ Rationale During a hospitalization for HF, a predischarge natriuretic peptide level can be useful to establish a postdischarge prognosis. NEW: Current recommendation reflects new observational studies. In patients with chronic HF, measurement of other clinically available tests, such as biomarkers of myocardial injury or fibrosis, may be considered for additive risk stratification. MODIFIED: 2013 recommendations have been combined into prognosis section, resulting in LOE change from A to BNR.

2017 Heart Failure Focused Update Treatment of HF Stages A Through D
Treatment of HF Stages A Through D Stage C
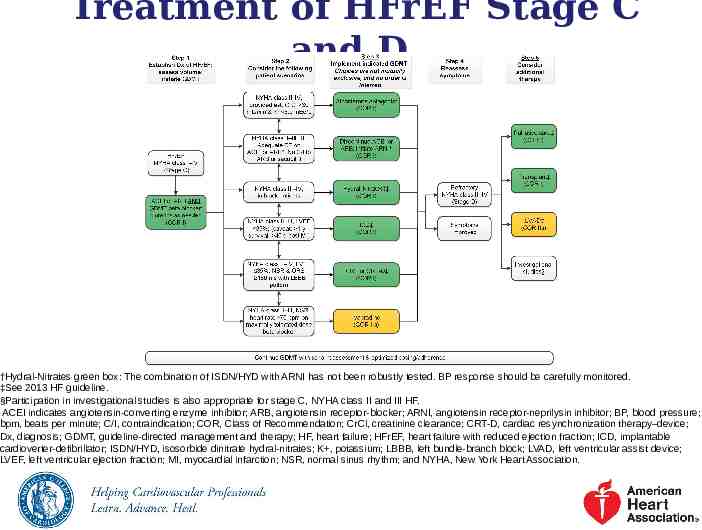
Treatment of HFrEF Stage C and D †Hydral-Nitrates green box: The combination of ISDN/HYD with ARNI has not been robustly tested. BP response should be carefully monitored. ‡See 2013 HF guideline. §Participation in investigational studies is also appropriate for stage C, NYHA class II and III HF. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor-blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BP, blood pressure; bpm, beats per minute; C/I, contraindication; COR, Class of Recommendation; CrCl, creatinine clearance; CRT-D, cardiac resynchronization therapy–device; Dx, diagnosis; GDMT, guideline-directed management and therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; ISDN/HYD, isosorbide dinitrate hydral-nitrates; K , potassium; LBBB, left bundle-branch block; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSR, normal sinus rhythm; and NYHA, New York Heart Association.
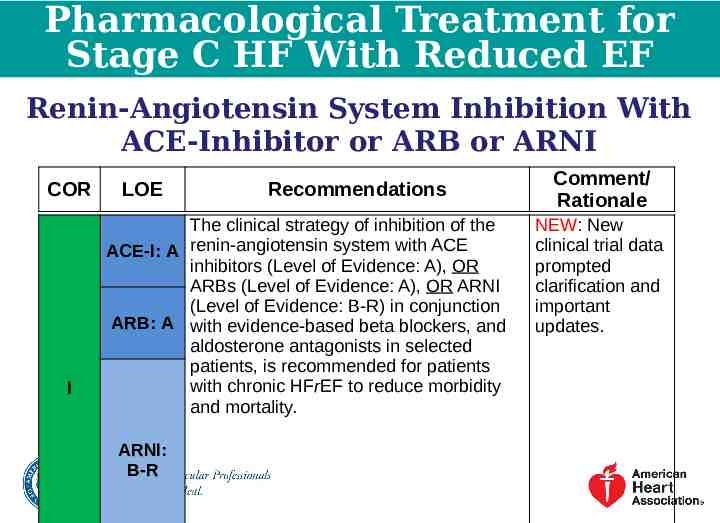
Pharmacological Treatment for Stage C HF With Reduced EF Renin-Angiotensin System Inhibition With ACE-Inhibitor or ARB or ARNI COR I LOE Recommendations The clinical strategy of inhibition of the ACE-I: A renin-angiotensin system with ACE inhibitors (Level of Evidence: A), OR ARBs (Level of Evidence: A), OR ARNI (Level of Evidence: B-R) in conjunction ARB: A with evidence-based beta blockers, and aldosterone antagonists in selected patients, is recommended for patients with chronic HFrEF to reduce morbidity and mortality. ARNI: B-R Comment/ Rationale NEW: New clinical trial data prompted clarification and important updates.
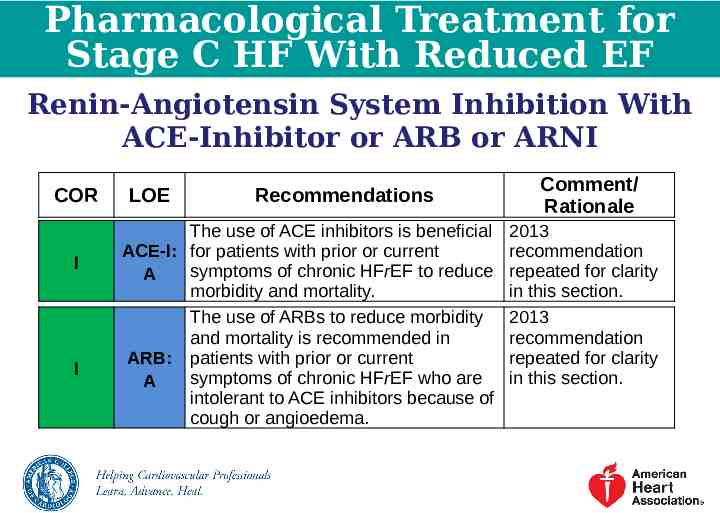
Pharmacological Treatment for Stage C HF With Reduced EF Renin-Angiotensin System Inhibition With ACE-Inhibitor or ARB or ARNI COR I I LOE Recommendations The use of ACE inhibitors is beneficial ACE-I: for patients with prior or current symptoms of chronic HFrEF to reduce A morbidity and mortality. The use of ARBs to reduce morbidity and mortality is recommended in ARB: patients with prior or current symptoms of chronic HFrEF who are A intolerant to ACE inhibitors because of cough or angioedema. Comment/ Rationale 2013 recommendation repeated for clarity in this section. 2013 recommendation repeated for clarity in this section.
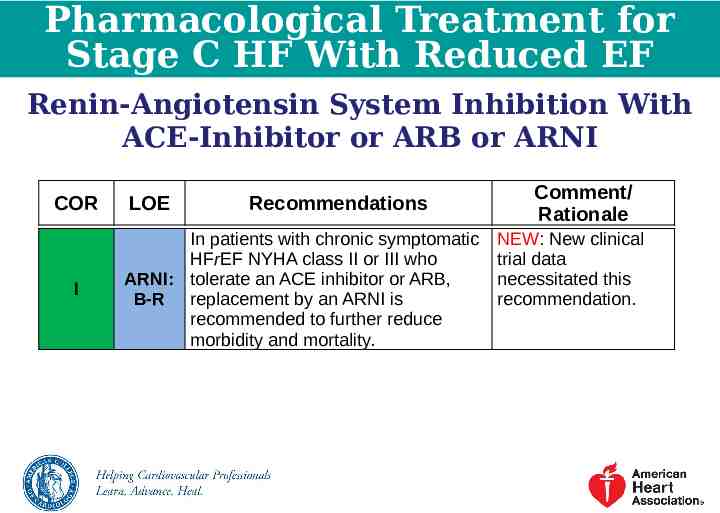
Pharmacological Treatment for Stage C HF With Reduced EF Renin-Angiotensin System Inhibition With ACE-Inhibitor or ARB or ARNI COR I LOE Recommendations In patients with chronic symptomatic HFrEF NYHA class II or III who ARNI: tolerate an ACE inhibitor or ARB, B-R replacement by an ARNI is recommended to further reduce morbidity and mortality. Comment/ Rationale NEW: New clinical trial data necessitated this recommendation.
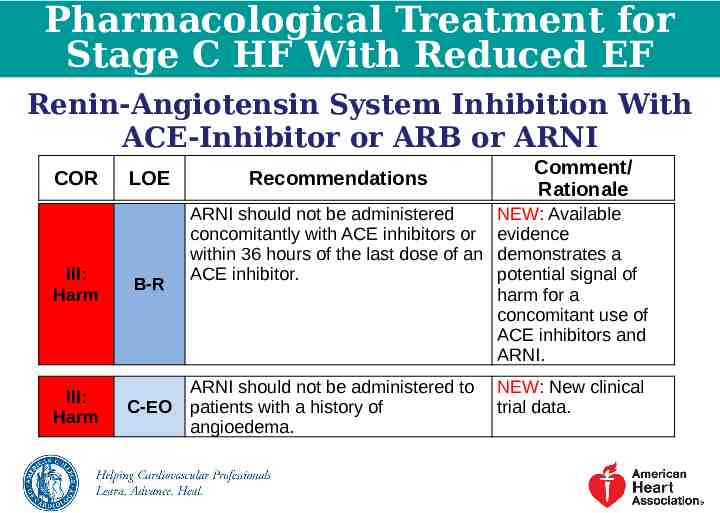
Pharmacological Treatment for Stage C HF With Reduced EF Renin-Angiotensin System Inhibition With ACE-Inhibitor or ARB or ARNI COR III: Harm III: Harm LOE B-R C-EO Recommendations Comment/ Rationale ARNI should not be administered concomitantly with ACE inhibitors or within 36 hours of the last dose of an ACE inhibitor. NEW: Available evidence demonstrates a potential signal of harm for a concomitant use of ACE inhibitors and ARNI. ARNI should not be administered to patients with a history of angioedema. NEW: New clinical trial data.
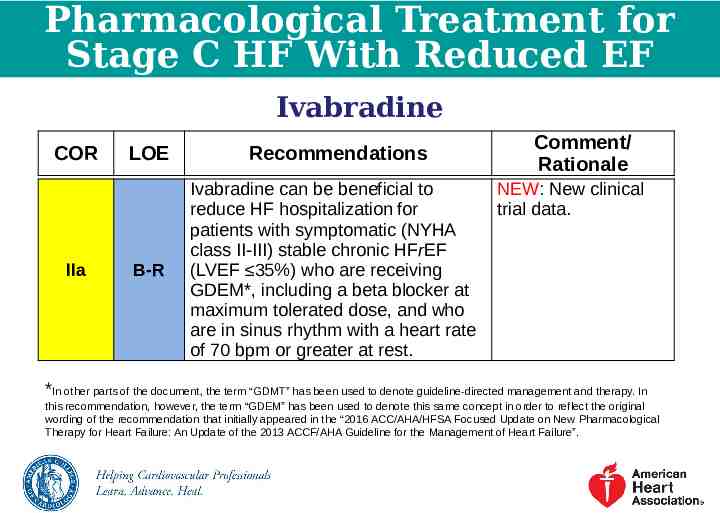
Pharmacological Treatment for Stage C HF With Reduced EF Ivabradine COR IIa LOE Recommendations B-R Ivabradine can be beneficial to reduce HF hospitalization for patients with symptomatic (NYHA class II-III) stable chronic HFrEF (LVEF 35%) who are receiving GDEM*, including a beta blocker at maximum tolerated dose, and who are in sinus rhythm with a heart rate of 70 bpm or greater at rest. Comment/ Rationale NEW: New clinical trial data. *In other parts of the document, the term “GDMT” has been used to denote guideline-directed management and therapy. In this recommendation, however, the term “GDEM” has been used to denote this same concept in order to reflect the original wording of the recommendation that initially appeared in the “2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure”.
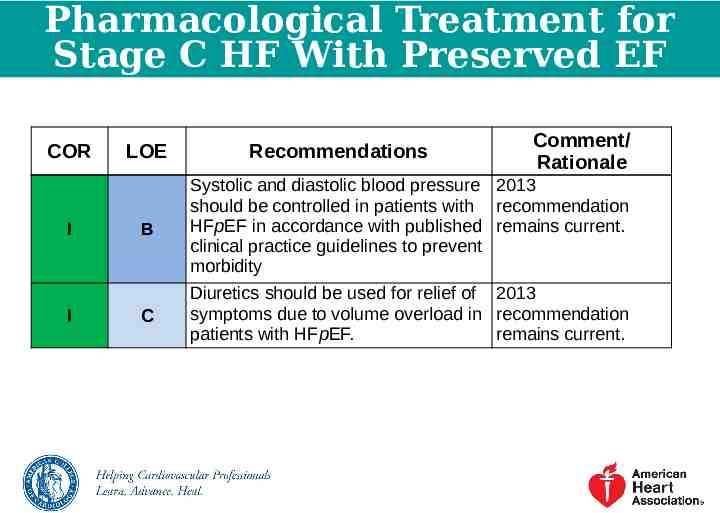
Pharmacological Treatment for Stage C HF With Preserved EF COR I I LOE Recommendations Comment/ Rationale B Systolic and diastolic blood pressure 2013 should be controlled in patients with recommendation HFpEF in accordance with published remains current. clinical practice guidelines to prevent morbidity C Diuretics should be used for relief of 2013 symptoms due to volume overload in recommendation patients with HFpEF. remains current.
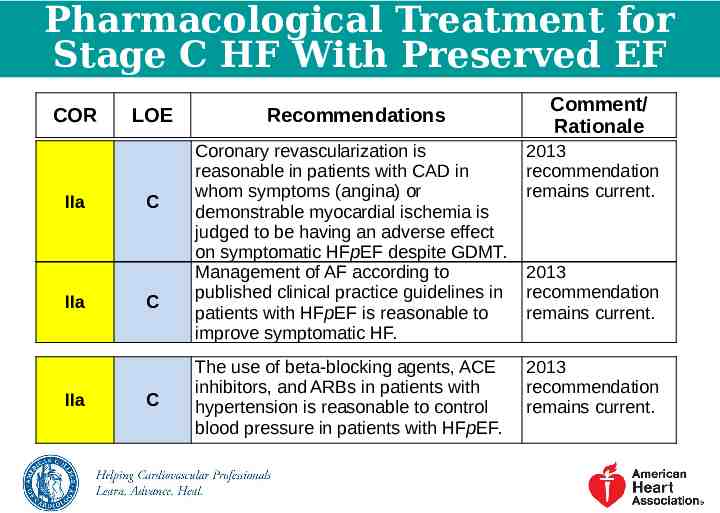
Pharmacological Treatment for Stage C HF With Preserved EF COR IIa IIa IIa LOE C C C Recommendations Comment/ Rationale Coronary revascularization is reasonable in patients with CAD in whom symptoms (angina) or demonstrable myocardial ischemia is judged to be having an adverse effect on symptomatic HFpEF despite GDMT. Management of AF according to published clinical practice guidelines in patients with HFpEF is reasonable to improve symptomatic HF. 2013 recommendation remains current. The use of beta-blocking agents, ACE inhibitors, and ARBs in patients with hypertension is reasonable to control blood pressure in patients with HFpEF. 2013 recommendation remains current. 2013 recommendation remains current.
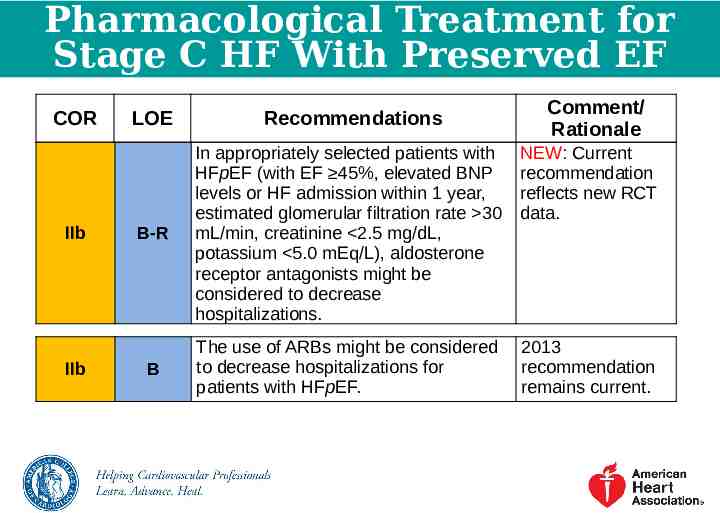
Pharmacological Treatment for Stage C HF With Preserved EF COR IIb IIb Comment/ Rationale LOE Recommendations NEW: Current recommendation reflects new RCT data. B-R In appropriately selected patients with HFpEF (with EF 45%, elevated BNP levels or HF admission within 1 year, estimated glomerular filtration rate 30 mL/min, creatinine 2.5 mg/dL, potassium 5.0 mEq/L), aldosterone receptor antagonists might be considered to decrease hospitalizations. B The use of ARBs might be considered to decrease hospitalizations for patients with HFpEF. 2013 recommendation remains current.
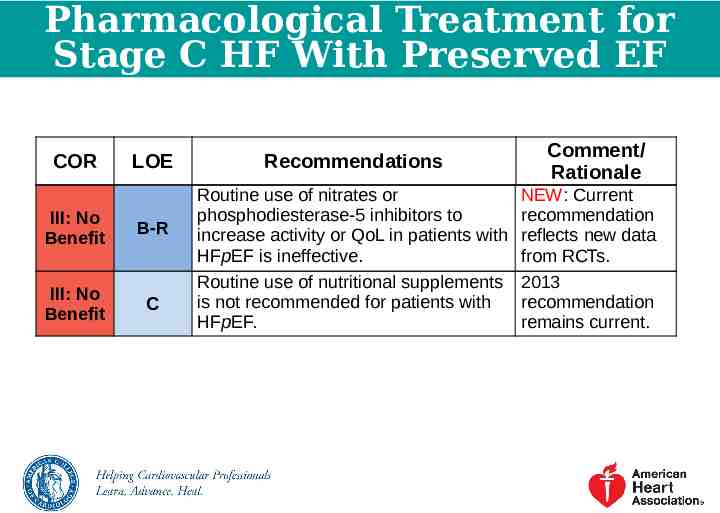
Pharmacological Treatment for Stage C HF With Preserved EF COR LOE III: No Benefit B-R III: No Benefit C Recommendations Routine use of nitrates or phosphodiesterase-5 inhibitors to increase activity or QoL in patients with HFpEF is ineffective. Routine use of nutritional supplements is not recommended for patients with HFpEF. Comment/ Rationale NEW: Current recommendation reflects new data from RCTs. 2013 recommendation remains current.

2017 Heart Failure Focused Update Important Comorbidities in HF
Important Comorbidities in HF Anemia
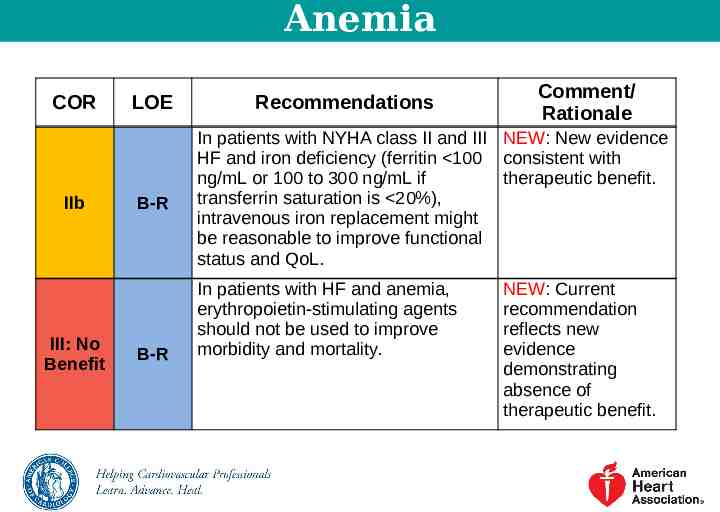
Anemia COR IIb III: No Benefit LOE B-R B-R Recommendations Comment/ Rationale In patients with NYHA class II and III NEW: New evidence HF and iron deficiency (ferritin 100 consistent with ng/mL or 100 to 300 ng/mL if therapeutic benefit. transferrin saturation is 20%), intravenous iron replacement might be reasonable to improve functional status and QoL. In patients with HF and anemia, erythropoietin-stimulating agents should not be used to improve morbidity and mortality. NEW: Current recommendation reflects new evidence demonstrating absence of therapeutic benefit.

Important Comorbidities in HF Hypertension (New Section)
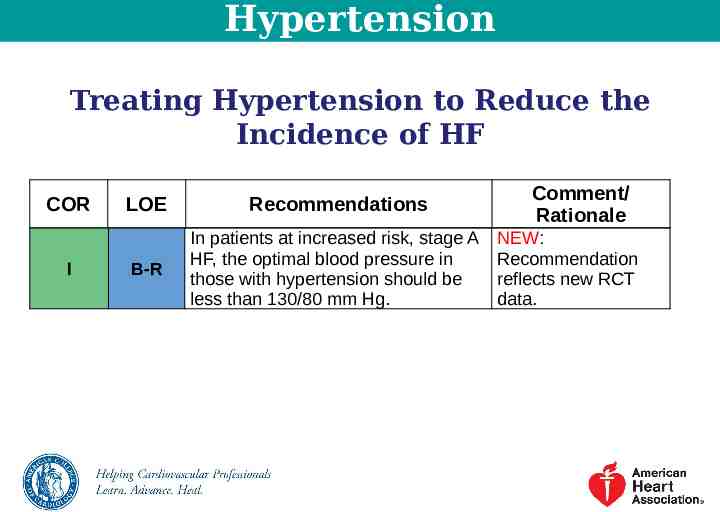
Hypertension Treating Hypertension to Reduce the Incidence of HF COR I LOE Recommendations B-R In patients at increased risk, stage A HF, the optimal blood pressure in those with hypertension should be less than 130/80 mm Hg. Comment/ Rationale NEW: Recommendation reflects new RCT data.
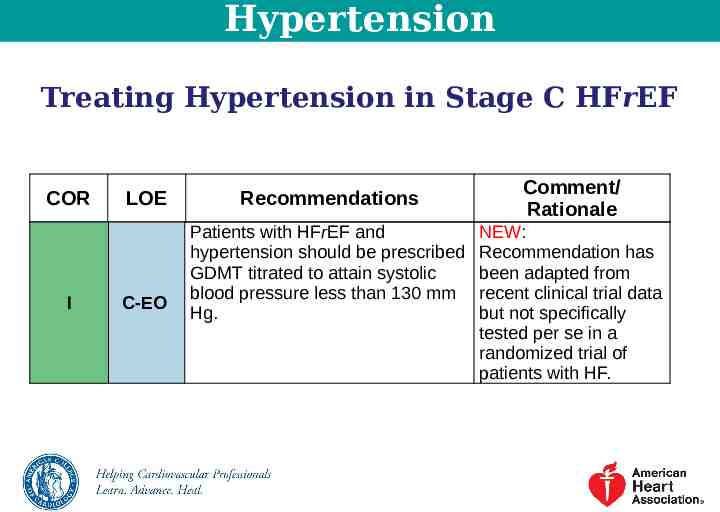
Hypertension Treating Hypertension in Stage C HFrEF COR I LOE C-EO Recommendations Patients with HFrEF and hypertension should be prescribed GDMT titrated to attain systolic blood pressure less than 130 mm Hg. Comment/ Rationale NEW: Recommendation has been adapted from recent clinical trial data but not specifically tested per se in a randomized trial of patients with HF.
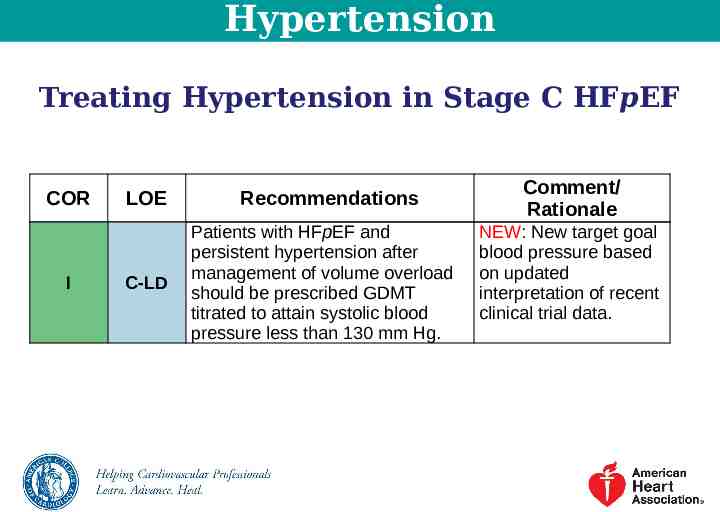
Hypertension Treating Hypertension in Stage C HFpEF COR I LOE C-LD Recommendations Patients with HFpEF and persistent hypertension after management of volume overload should be prescribed GDMT titrated to attain systolic blood pressure less than 130 mm Hg. Comment/ Rationale NEW: New target goal blood pressure based on updated interpretation of recent clinical trial data.

Important Comorbidities in HF Sleep Disorders (Moved from Section 7.3.1.4, Treatment of Sleep Disorders in the 2013 HF guideline)
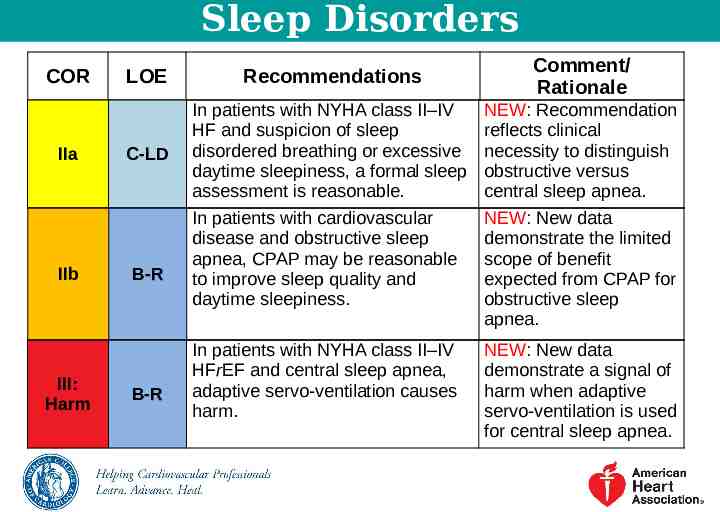
Sleep Disorders COR IIa IIb III: Harm LOE Recommendations Comment/ Rationale C-LD In patients with NYHA class II–IV HF and suspicion of sleep disordered breathing or excessive daytime sleepiness, a formal sleep assessment is reasonable. NEW: Recommendation reflects clinical necessity to distinguish obstructive versus central sleep apnea. In patients with cardiovascular disease and obstructive sleep apnea, CPAP may be reasonable to improve sleep quality and daytime sleepiness. NEW: New data demonstrate the limited scope of benefit expected from CPAP for obstructive sleep apnea. In patients with NYHA class II–IV HFrEF and central sleep apnea, adaptive servo-ventilation causes harm. NEW: New data demonstrate a signal of harm when adaptive servo-ventilation is used for central sleep apnea. B-R B-R






