VMD Pharmaceutical Industry Customer Satisfaction Summary Q1 2022
65 Slides2.75 MB

VMD Pharmaceutical Industry Customer Satisfaction Summary Q1 2022 Survey MV006· Q1 2022· VMD Pharmaceutical Industry Customer Satisfaction Survey

BACKGROUND The Veterinary Medicines Directorate (VMD) has, since 2004, surveyed its Marketing Authorisation holders with a view to measuring customer satisfaction. As this survey has been carried out by the same agency team lead since 2004, VMD has been able to use this survey as a benchmarking study by which they can monitor their performance. The previous wave was conducted in 2017/2018 and comprised: A quantitative survey 131 responses received Multiple responses per company were permitted Qualitative survey Post analysis, 8 follow-up qualitative interviews by telephone Duration between 5 minutes and 21 minutes Covered, on average, two areas of interest to the VMD in which the VMD had been scored relatively lowly by these respondents MV006. VMD CSS. October 2021

BACKGROUND For this wave, the methodology was flipped with qualitative interviews being conducted BEFORE the quantitative phase. This was, in fact, the original methodology but latterly, the market was considered to be relatively stable and perhaps did not warrant full, in-depth interviews. The main reason for reverting to the original methodology was that the VMD is operating in a period of considerable change: New Chief Executive Officer at VMD Changes to the Veterinary Medicines Regulations Change in EU Regulations Brexit and the milestones It was felt that, more than ever, customers may welcome the opportunity to reflect backwards and forwards in relation to what they feel the VMD has handled well and what they feel could be done going forward to support them. This report summarises the findings of both the qualitative and quantitative phases of the Customer Satisfaction Survey. MV006. VMD CSS. October 2021

OBJECTIVES: PHASE 1 Qualitative research To understand the perceived strengths and weaknesses of the VMD To gain an overview of the extent to which the Veterinary Medicines Digital Services platform is meeting customer needs To understand the current level of satisfaction with the VMD and how, if at all, this has changed over the past 2 years To gain industry feedback on what they would see as the priorities for the CEO in the short and medium term MV006. VMD CSS. October 2021

METHODOLOGY: PHASE 1 Eight in-depth interviews were undertaken, by Zoom, in Q3 2021. Although invitations to take part in the qualitative research were sent to a broad cross-section of customers, the resultant sample of UKbased Regulatory Affairs personnel, comprised mainly companies who: Were members of NOAH Had 50 Marketing Authorisations MV006. VMD CSS. October 2021

SUMMARY: PHASE 1 A very high level of satisfaction with vmd Industry has a very high level of satisfaction with VMD. Industry recognises that these are challenging times and feels that the VMD has risen to the challenge. The VMD is seen as helpful, pragmatic and / or collaborative. “They [VMD] have dealt with a difficult time you do get that feeling of collaboration and working together as a partnership [mutual respect]” MV006. VMD CSS. October 2021

SUMMARY PHASE 1: STRENGTHS OF VMD Strengths of the VMD The key strengths of the VMD centred on: Interpersonal skills, attitudes and behaviours Expertise Communication The VMDS (although further improvements would be welcomed) The value of the VMD collaborating with the current list non-EU Regulators was debated. This was, however, outside their remit for many. MV006. VMD CSS. October 2021

SUMMARY PHASE 1: AREAS FOR IMPROVEMENT VMD areas for improvement The key areas for improvement centred on: Lack of information in relation to the air gap and the NI Protocol Modifications to the VMDS Adherence to / visibility on timelines when applying in parallel Inconsistencies in advice Duplication of fees (NI) Duplication of emails during multiple batch release Duplication of efforts in relation to the QRD Gold plating resulting in delays / additional work Hampering UK access to novel products through lack of creativity / flexibility in relation to assessing novel products and / or granting an ATC MV006. VMD CSS. October 2021

SUMMARY PHASE 1: CUSTOMER PRIORITIES Priorities Moving forward, industry felt that the key priorities for the CEO in the short term should include: Managing and communicating in relation to the air gap – considered urgent Managing and communicating on the NI Protocol Seeking Virgin Plastics tax exemption Managing staff changes In the medium to long term, industry felt that the VMD should prioritise: The availability of medicines Collaboration opportunities with more / different non-EU Regulators Decreasing the administrative burden MV006. VMD CSS. October 2021

SUMMARY PHASE 1: IMAGE If the VMD were a type of car: It would mostly likely be a Skoda or Volvo Key themes: Reliable, trustworthy, no frills (economical), gold standard/high quality MV006. VMD CSS. October 2021

OBJECTIVES: PHASE 2 Online survey Whilst the overall objectives of the survey remain to measure customer satisfaction amongst MA holders, VMD are particularly interested in the following: The % of respondents who give a score of good (4/5) or excellent (5/5) against a specific target for each of the teams at the VMD (KPI: On average for each of the VMD teams their work is rated as good / excellent (scale 1-5 with 4 good and 5 excellent) by not less than 70% of customers A comparison of the % of respondents giving a score of good / excellent with the previous wave An understanding of the issues are behind any poor scores with a view to being able to pursue improvements that make a difference to their customers MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

METHODOLOGY: PHASE 2 An online survey was conducted in Q1 2022. This survey comprised elements raised in the qualitative phase (Phase 1) plus additional areas of interest to VMD in 2022. The survey was aimed at companies that have marketing and / or manufacturing authorisations and is someone who has had personal experience of relevant departments at the VMD within the last 12 months. Respondents were invited, and reminded, by VMD to participate in the survey. Respondents were able to partially complete and go back at a later stage to complete the survey before submitting The survey remained open for three weeks 88 responses received, compared with the previous wave of 131 Multiple responses per company permitted MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
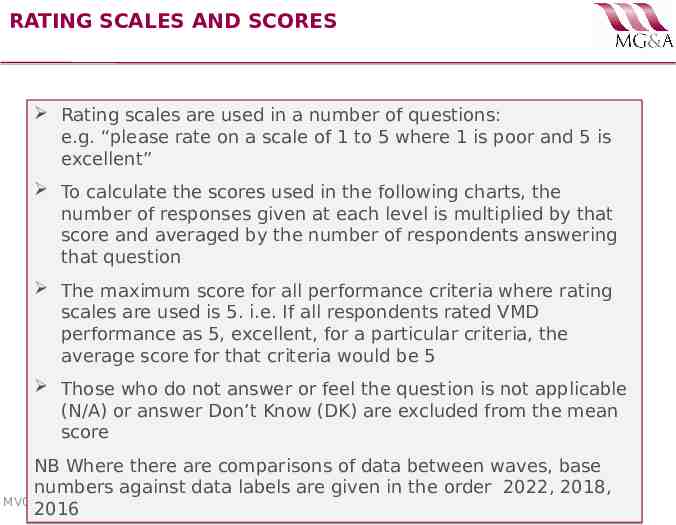
RATING SCALES AND SCORES Rating scales are used in a number of questions: e.g. “please rate on a scale of 1 to 5 where 1 is poor and 5 is excellent” To calculate the scores used in the following charts, the number of responses given at each level is multiplied by that score and averaged by the number of respondents answering that question The maximum score for all performance criteria where rating scales are used is 5. i.e. If all respondents rated VMD performance as 5, excellent, for a particular criteria, the average score for that criteria would be 5 Those who do not answer or feel the question is not applicable (N/A) or answer Don’t Know (DK) are excluded from the mean score NB Where there are comparisons of data between waves, base numbers against data labels are given in the order 2022, 2018, MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey 2016

ANALYSIS SCORING SYSTEM USED Excellent % of respondents rating (4 5) 80% Good % of respondents rating (4 5) 60% - 80% Review % of respondents rating (4 5) 40% - 60% MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
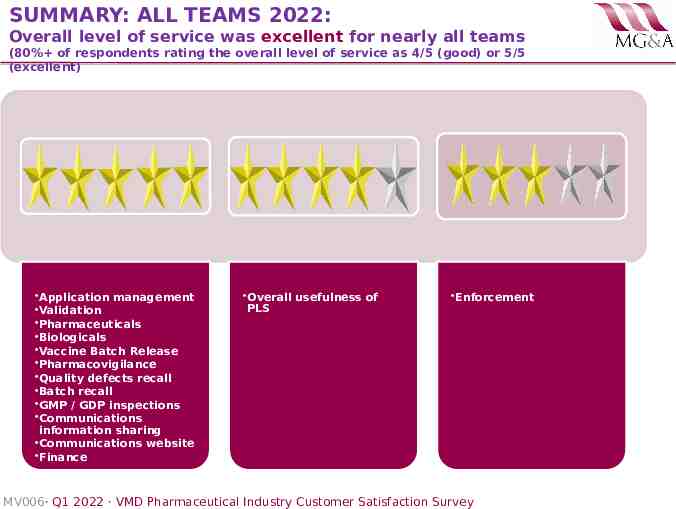
SUMMARY: ALL TEAMS 2022: Overall level of service was excellent for nearly all teams (80% of respondents rating the overall level of service as 4/5 (good) or 5/5 (excellent) Application management Validation Pharmaceuticals Biologicals Vaccine Batch Release Pharmacovigilance Quality defects recall Batch recall GMP / GDP inspections Communications information sharing Communications website Finance Overall usefulness of PLS Enforcement MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

APPLICATION MANAGEMENT (n 52 or less) How would you rate the service you have received from APPLICATION MANAGEMENT (formerly Licensing Administration), specifically in the last 12 MONTHS? By Application Management we mean the team that provides: · Advice on regulatory issues · Advice on application procedures and processes including submission · Advice following the completion of EU referrals · Validation of renewal applications and administrative variations · Issuing of marketing authorisation documentation. We are not referring to validation enquiries, validation documentation or safety, quality, efficacy questions at this point. MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: APPLICATION MANAGEMENT 2022: Two thirds of parameters rated excellent Overall level of service Knowledge of staff responding to enquiries Approachability Helpfulness of staff Usefulness of advice given Speed of response to enquiries Consistency of advice given between staff MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
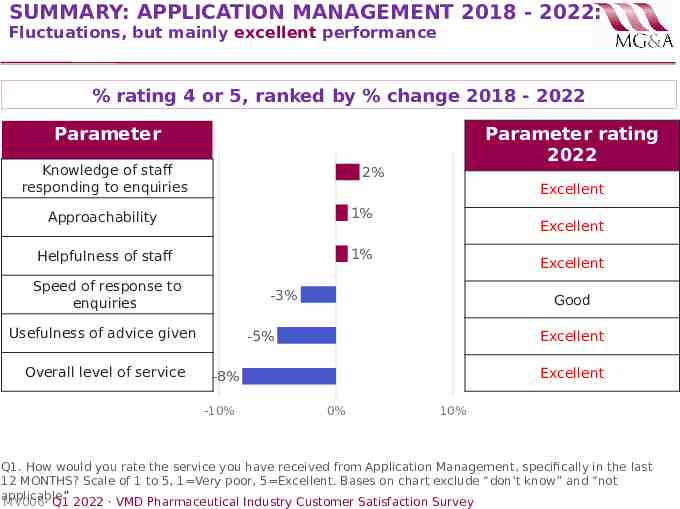
SUMMARY: APPLICATION MANAGEMENT 2018 - 2022: Fluctuations, but mainly excellent performance % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Knowledge of staff responding to enquiries 2% Excellent Approachability 1% Helpfulness of staff 1% Speed of response to enquiries Excellent Excellent -3% Usefulness of advice given Overall level of service Parameter rating 2022 Good -5% Excellent Excellent -8% -10% 0% 10% Q1. How would you rate the service you have received from Application Management, specifically in the last 12 MONTHS? Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

VALIDATION PERFORMED BY APPLICATION MANAGEMENT (FORMERLY THE GENERAL ASSESSMENT TEAM (GAT)) (n 22 or less) The next two questions refer to the checking process undertaken following the receipt of an application at the VMD. It relates to pharmaceutical and Biological applications and specifically to new marketing authorisations and most variations. MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: VALIDATION 2022: All parameters excellent Overall level of service Flexibility of approach/willingness to listen to a reasoned argument Knowledge of staff responding to enquiries Helpfulness of staff Clarity of the points included in validation deferral letters Approachability of staff Consistency of advice given Speed of response to enquiries Usefulness of advice given Ease of identifying the correct person to speak to in this area MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
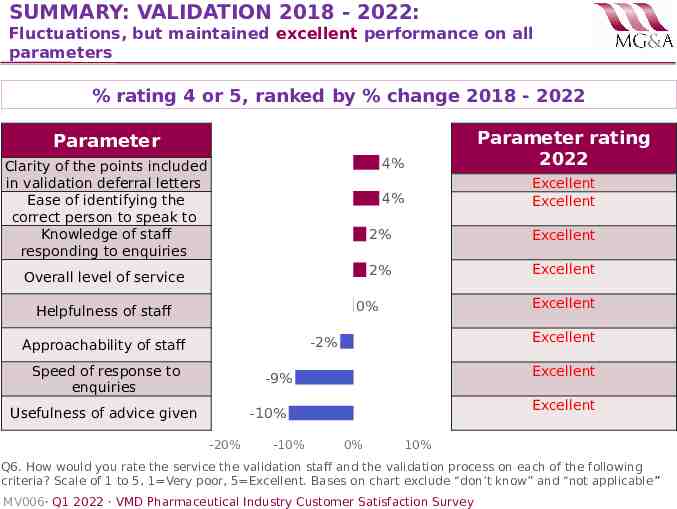
SUMMARY: VALIDATION 2018 - 2022: Fluctuations, but maintained excellent performance on all parameters % rating 4 or 5, ranked by % change 2018 - 2022 4% Parameter rating 2022 4% Excellent Excellent Parameter Clarity of the points included in validation deferral letters Ease of identifying the correct person to speak to Knowledge of staff responding to enquiries Overall level of service 2% Excellent 2% Excellent Excellent 0% Helpfulness of staff Excellent -2% Approachability of staff Speed of response to enquiries Excellent -9% Usefulness of advice given Excellent -10% -20% -10% 0% 10% Q6. How would you rate the service the validation staff and the validation process on each of the following criteria? Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

PRODUCT LITERATURE STANDARD (PLS) (n 19 or less) The following questions relate to the Product Literature Standard (PLS). The Standard is meant as a guide for applicants when they are creating mock-ups for submission to the VMD. MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
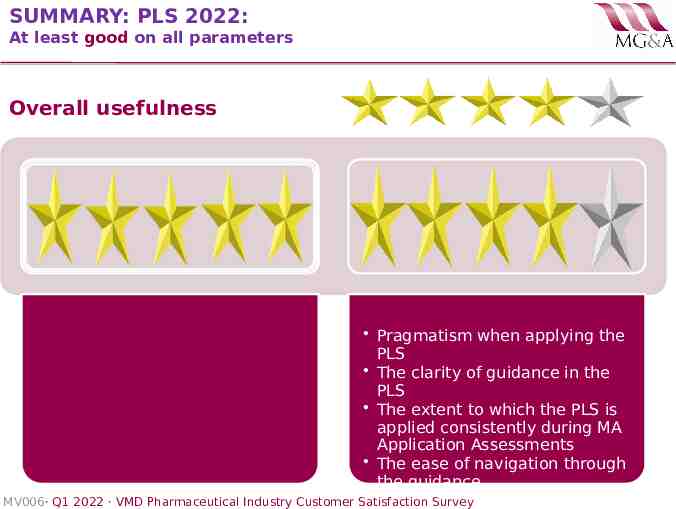
SUMMARY: PLS 2022: At least good on all parameters Overall usefulness Pragmatism when applying the PLS The clarity of guidance in the PLS The extent to which the PLS is applied consistently during MA Application Assessments The ease of navigation through the guidance MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
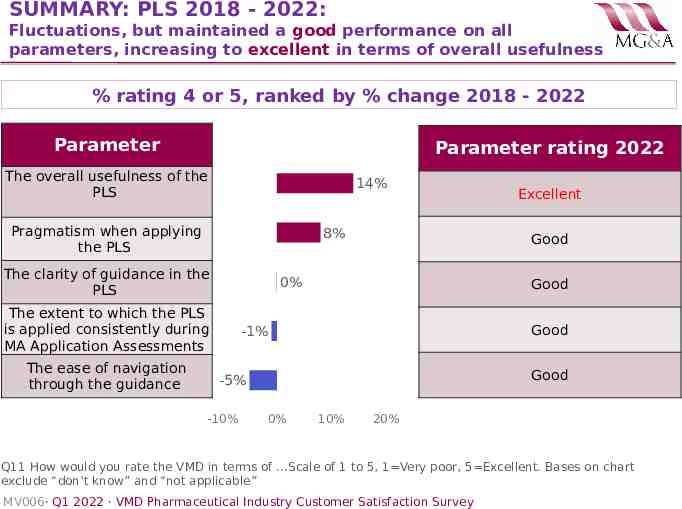
SUMMARY: PLS 2018 - 2022: Fluctuations, but maintained a good performance on all parameters, increasing to excellent in terms of overall usefulness % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Parameter rating 2022 The overall usefulness of the PLS 14% Pragmatism when applying the PLS 8% The clarity of guidance in the PLS The ease of navigation through the guidance Good 0% The extent to which the PLS is applied consistently during MA Application Assessments Good Good -1% Good -5% -10% Excellent 0% 10% 20% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

PHARMACEUTICALS (n 19 or less) MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: PHARMACEUTICALS 2022: Excellent on all parameters Overall level of service Consistency of approach between assessors Pragmatism / willingness to listen to a reasonable alternative view Ease of identifying the correct person to speak to in this area Knowledge of staff responding to enquiries Approachability Relevance of q's asked by pharmaceutical safety assessors Usefulness of advice Helpfulness of staff Relevance of q's asked by pharmaceutical eco-tox assessors Relevance of questions asked by pharmaceutical quality assessors Speed of response to enquiries Relevance of q's asked by pharmaceutical efficacy assessors MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
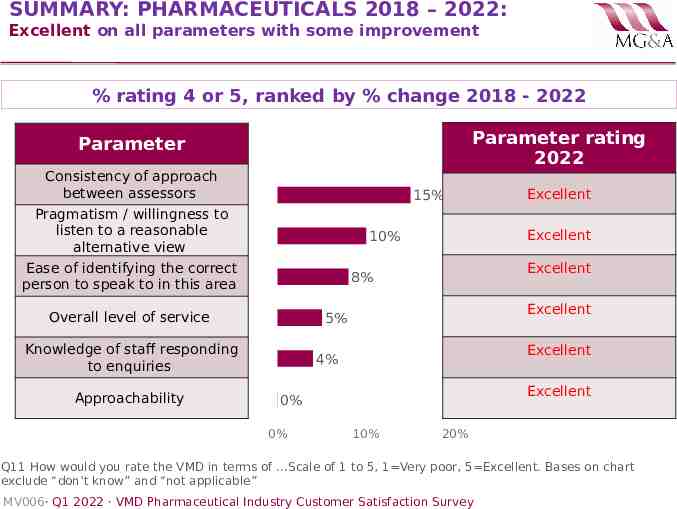
SUMMARY: PHARMACEUTICALS 2018 – 2022: Excellent on all parameters with some improvement % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter Consistency of approach between assessors 15% Pragmatism / willingness to listen to a reasonable alternative view Excellent 8% Overall level of service Excellent 5% Knowledge of staff responding to enquiries Approachability Excellent 10% Ease of identifying the correct person to speak to in this area Excellent 4% Excellent 0% 0% Excellent 10% 20% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
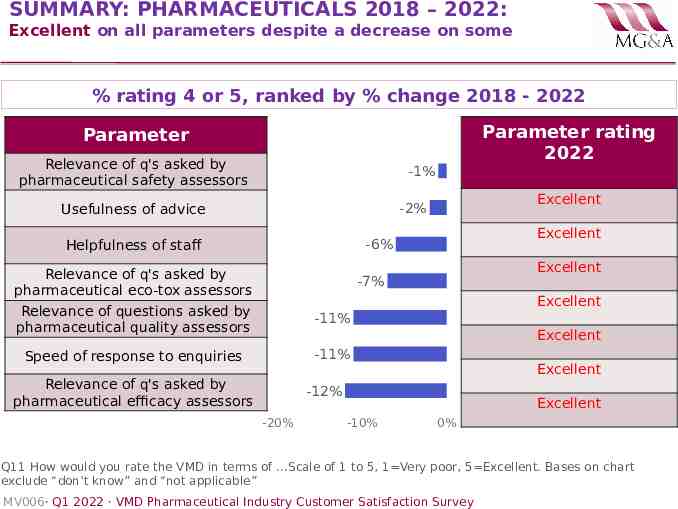
SUMMARY: PHARMACEUTICALS 2018 – 2022: Excellent on all parameters despite a decrease on some % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter Relevance of q's asked by pharmaceutical safety assessors -1% Excellent -2% Usefulness of advice Excellent -6% Helpfulness of staff Relevance of q's asked by pharmaceutical eco-tox assessors Relevance of questions asked by pharmaceutical quality assessors Excellent -7% Excellent -11% Excellent -11% Speed of response to enquiries Relevance of q's asked by pharmaceutical efficacy assessors Excellent -12% -20% Excellent -10% 0% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

BIOLOGICALS (n 11 or less) MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base! Caution low base
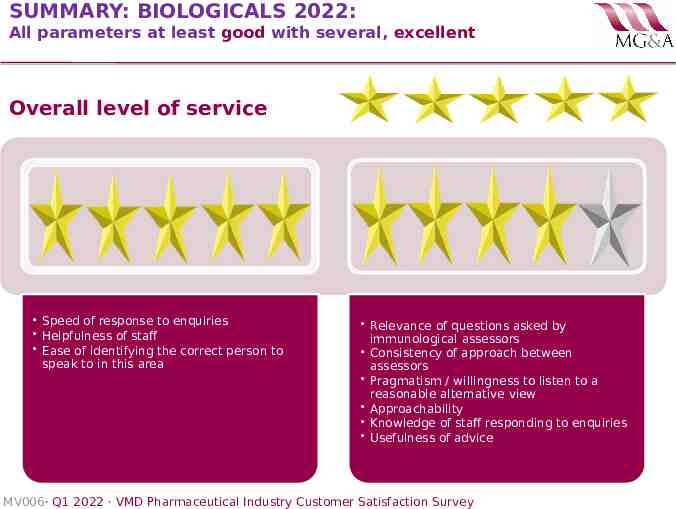
SUMMARY: BIOLOGICALS 2022: All parameters at least good with several, excellent Overall level of service Speed of response to enquiries Helpfulness of staff Ease of identifying the correct person to speak to in this area Relevance of questions asked by immunological assessors Consistency of approach between assessors Pragmatism / willingness to listen to a reasonable alternative view Approachability Knowledge of staff responding to enquiries Usefulness of advice MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
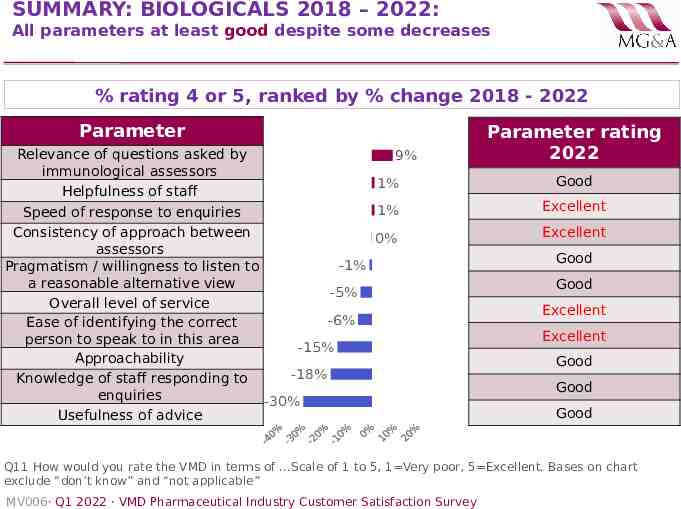
SUMMARY: BIOLOGICALS 2018 – 2022: All parameters at least good despite some decreases % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Relevance of questions asked by immunological assessors Helpfulness of staff 9% 1% 1% Speed of response to enquiries Consistency of approach between 0% assessors -1% Pragmatism / willingness to listen to a reasonable alternative view -5% Overall level of service -6% Ease of identifying the correct person to speak to in this area -15% Approachability -18% Knowledge of staff responding to enquiries -30% Usefulness of advice Parameter rating 2022 Good Excellent Excellent Good Good Excellent Excellent Good Good Good Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

VACCINE BATCH RELEASE (n 12 or less) The batch release scheme is a digital service with a target of 10 days to complete an application, although the VMD aims to deal with requests in shorter time frame. MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base! Caution low base

SUMMARY: VACCINE BATCH RELEASE 2022: Overall level of service Quality of issued documentation Advice provided Timescales for the process MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
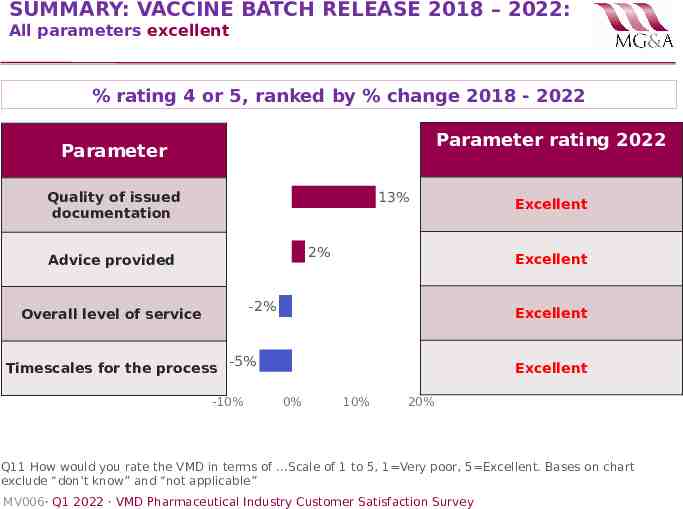
SUMMARY: VACCINE BATCH RELEASE 2018 – 2022: All parameters excellent % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter Quality of issued documentation 13% 2% Advice provided Excellent -2% Overall level of service Timescales for the process Excellent -5% -10% Excellent Excellent 0% 10% 20% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

PHARMACOVIGILANCE (n 21 or less) MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: PHARMACOVIGILANCE 2022: Excellent on all parameters Overall level of service Relevance of questions relating to Adverse Event reports Knowledge of staff responding to enquiries Pragmatism/willingness to listen to a reasonable alternative view Consistency of advice given Usefulness of advice Approachability Helpfulness of staff Consistency of approach between assessors Relevance of questions asked by PSUR assessors Speed of response to enquiries Ease of identifying the correct person to speak to in this area Relevance of questions asked by assessors during applications/renewals MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
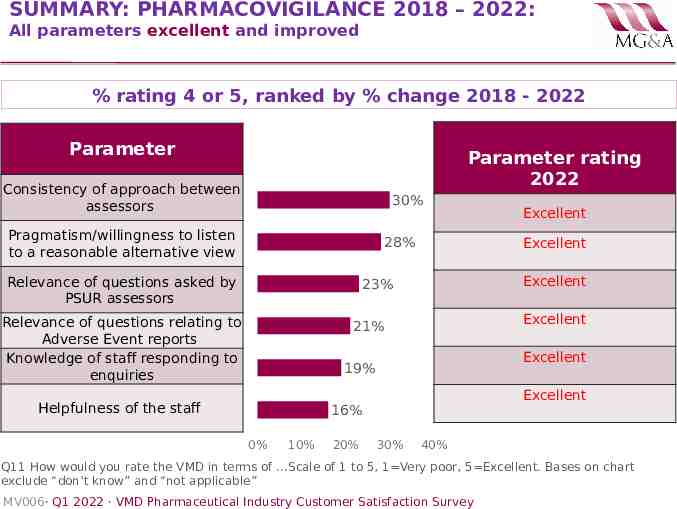
SUMMARY: PHARMACOVIGILANCE 2018 – 2022: All parameters excellent and improved % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Parameter rating 2022 Consistency of approach between assessors 30% Pragmatism/willingness to listen to a reasonable alternative view 28% Relevance of questions asked by PSUR assessors Excellent Excellent 23% Relevance of questions relating to Adverse Event reports Knowledge of staff responding to enquiries Excellent 21% Excellent 19% Helpfulness of the staff Excellent 16% 0% 10% 20% Excellent 30% 40% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
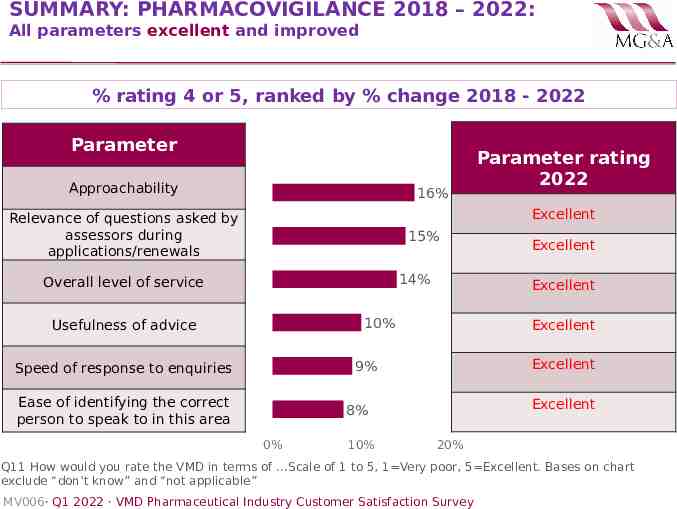
SUMMARY: PHARMACOVIGILANCE 2018 – 2022: All parameters excellent and improved % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Approachability 16% Parameter rating 2022 Excellent Relevance of questions asked by assessors during applications/renewals 15% 14% Overall level of service Excellent 10% Usefulness of advice Excellent Excellent 9% Speed of response to enquiries Ease of identifying the correct person to speak to in this area Excellent 8% 0% 10% Excellent 20% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

QUALITY DEFECT REPORTING AND BATCH RECALL (n 14 or less) MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base
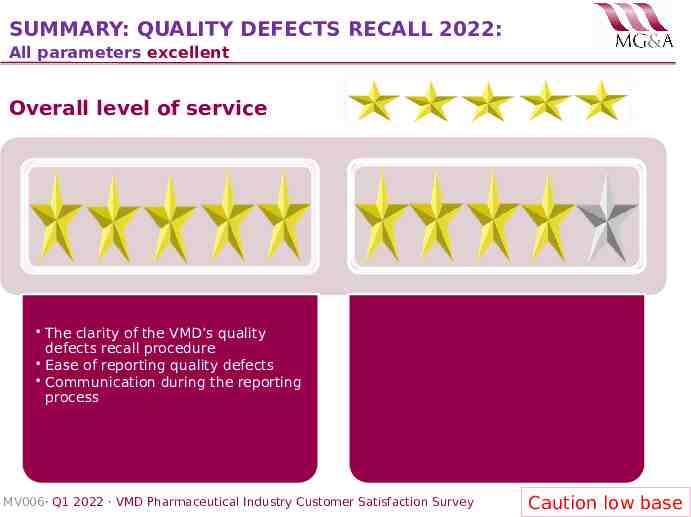
SUMMARY: QUALITY DEFECTS RECALL 2022: All parameters excellent Overall level of service The clarity of the VMD’s quality defects recall procedure Ease of reporting quality defects Communication during the reporting process MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base
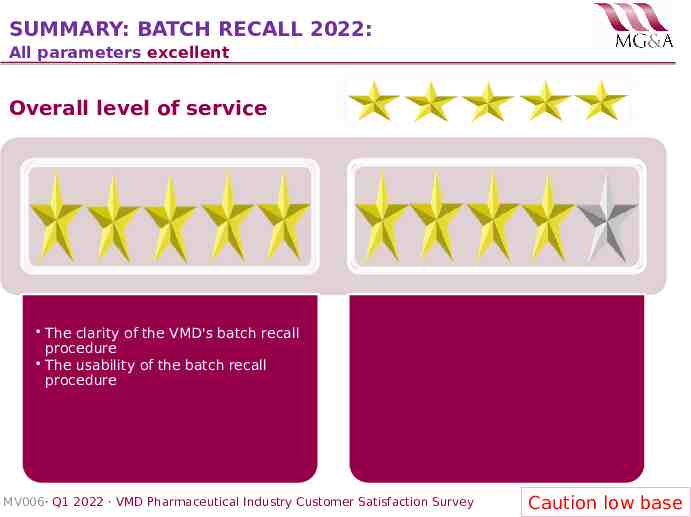
SUMMARY: BATCH RECALL 2022: All parameters excellent Overall level of service The clarity of the VMD's batch recall procedure The usability of the batch recall procedure MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base
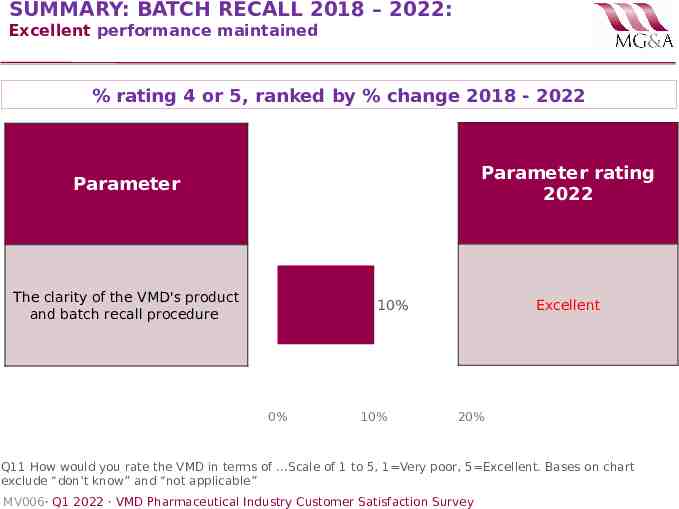
SUMMARY: BATCH RECALL 2018 – 2022: Excellent performance maintained % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter The clarity of the VMD's product and batch recall procedure Excellent 10% 0% 10% 20% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

GMP OR GDP INSPECTIONS Thinking now of Good Manufacturing Practice (GMP) or Wholesale Dealer (GDP) inspections performed by the VMD's inspectors including Schedule 6 inspections (Small Animal Exemption scheme, now called Exemptions for Small Pet Animals): MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: GMP & GDP INSPECTIONS 2022: All parameters excellent Overall level of service Conduct of the inspection The advice provide Timescale for the process Quality of the inspection report Clarity of the issues identified Quality of the issued documentation Organisational aspects MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
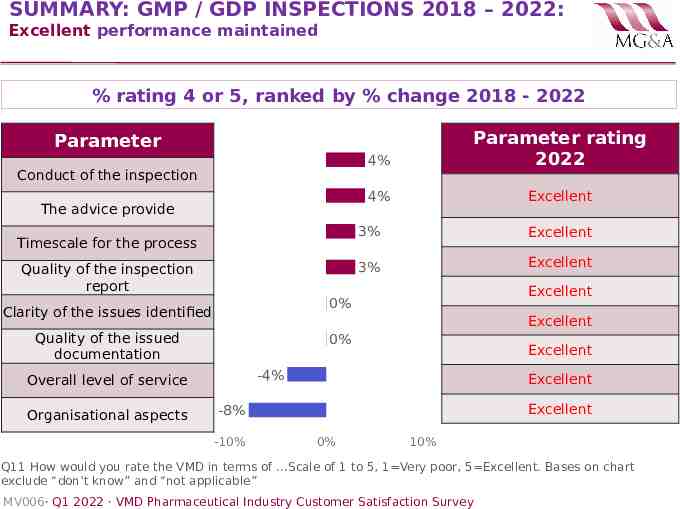
SUMMARY: GMP / GDP INSPECTIONS 2018 – 2022: Excellent performance maintained % rating 4 or 5, ranked by % change 2018 - 2022 4% Parameter rating 2022 4% Excellent Parameter Conduct of the inspection The advice provide Timescale for the process Quality of the inspection report Excellent 3% Excellent Excellent 0% Clarity of the issues identified Excellent Quality of the issued documentation 0% Excellent -4% Overall level of service Organisational aspects 3% Excellent Excellent -8% -10% 0% 10% Q11 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

ENFORCEMENT Enforcement is the department which is responsible for dealing with illegal sales or use of veterinary medicines MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base

SUMMARY: ENFORCEMENT 2022: Review, although at least satisfactory for 82% Overall level of satisfaction MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey Caution low base
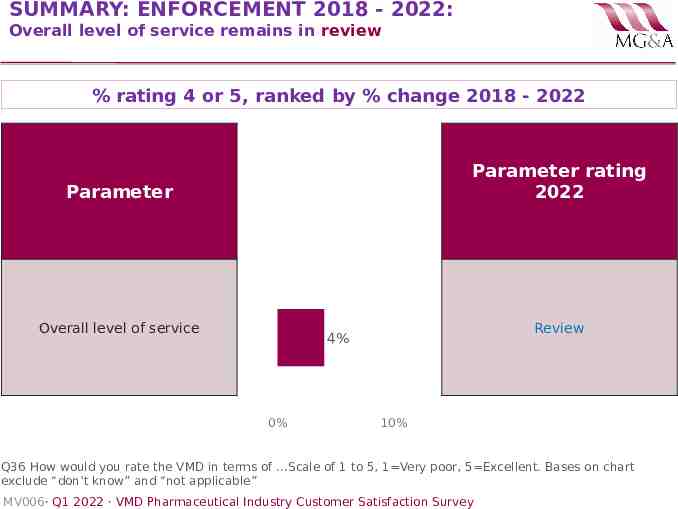
SUMMARY: ENFORCEMENT 2018 - 2022: Overall level of service remains in review % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter Overall level of service Review 4% 0% 10% Q36 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

COMMUNICATIONS MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

COMMUNICATIONS: CHARTER ON ENQUIRIES VMD's Service Standards for enquiries Enquiries and requests for information We aim to: respond to enquiries and complaints within 15 working days reply to Freedom of Information requests within 20 working days Sometimes we may need longer to respond, for example if you are asking for complex information or if we need to involve a third party. If we are unable to respond within these timescales, we will let you know. We will always try to answer your telephone calls promptly and respond to questions arising from them within 5 working days. If we cannot provide a complete answer within that time, we will let you know why. Where the first person you speak to cannot answer your query, we will ensure that someone who can deal with it calls you back, within 2 working days. If we are away from the office when you call us, we will ensure that you are told: * when we will be back; and * who you can contact in our absence. When you leave a message, we will call you back, or ensure that someone able to deal with your query calls you, within two working days of our return to the office. MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: COMMUNICATIONS INFORMATION SHARING ACTIVITIES 2022: Excellent across all parameters Overall usefulness of the information sharing activities Gov.uk news items Annual open information event Monthly update bulletin email Quarterly MAVIS The Information Hub MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
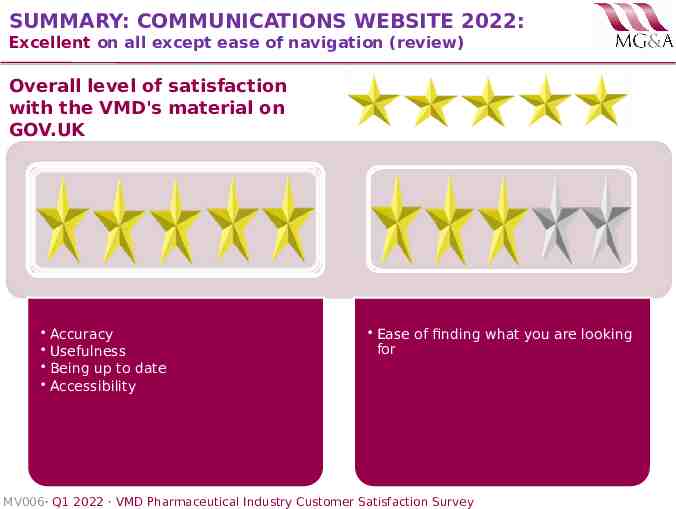
SUMMARY: COMMUNICATIONS WEBSITE 2022: Excellent on all except ease of navigation (review) Overall level of satisfaction with the VMD's material on GOV.UK Accuracy Usefulness Being up to date Accessibility Ease of finding what you are looking for MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
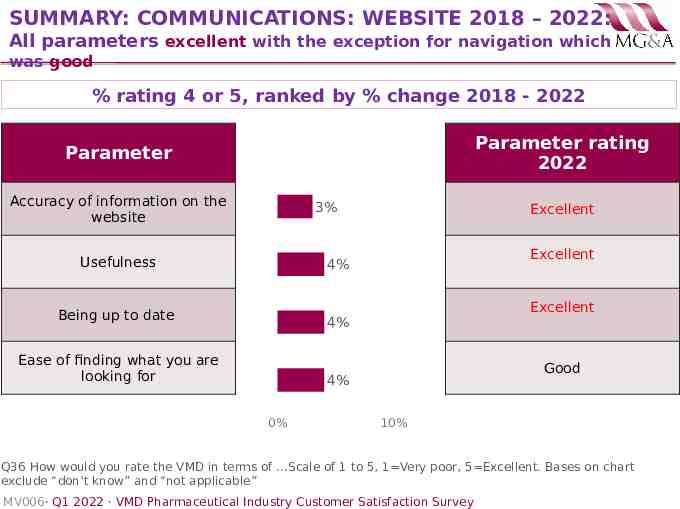
SUMMARY: COMMUNICATIONS: WEBSITE 2018 – 2022: All parameters excellent with the exception for navigation which was good % rating 4 or 5, ranked by % change 2018 - 2022 Parameter rating 2022 Parameter Accuracy of information on the website 3% Usefulness Excellent Excellent 4% Being up to date Excellent 4% Ease of finding what you are looking for Good 4% 0% 10% Q36 How would you rate the VMD in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

FINANCE (n 17) FINANCE MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
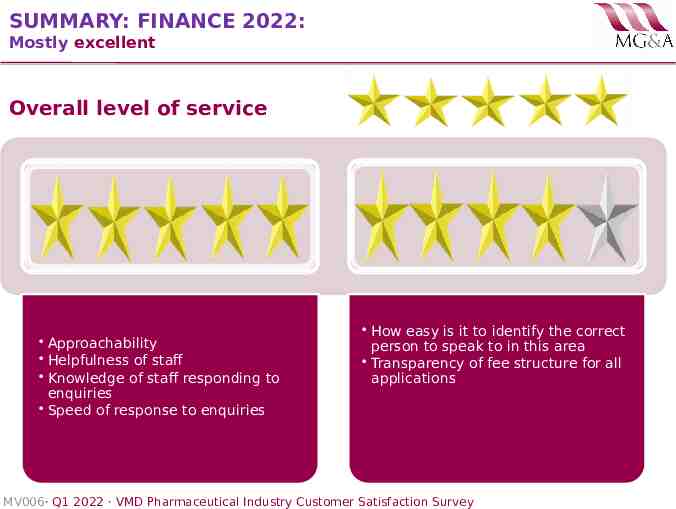
SUMMARY: FINANCE 2022: Mostly excellent Overall level of service Approachability Helpfulness of staff Knowledge of staff responding to enquiries Speed of response to enquiries How easy is it to identify the correct person to speak to in this area Transparency of fee structure for all applications MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
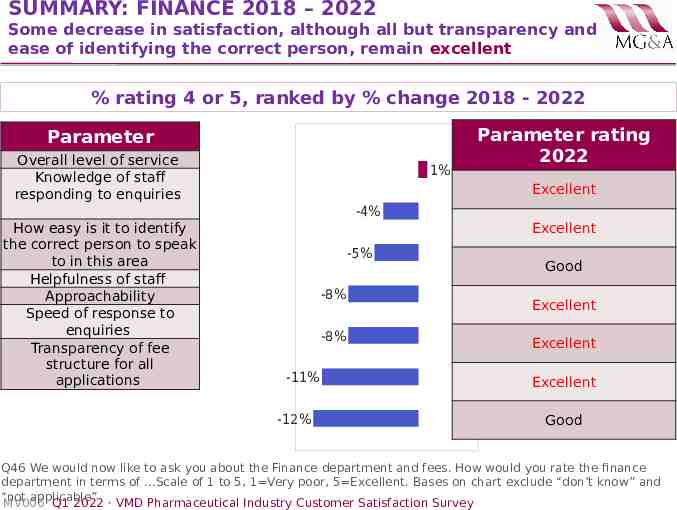
SUMMARY: FINANCE 2018 – 2022 Some decrease in satisfaction, although all but transparency and ease of identifying the correct person, remain excellent % rating 4 or 5, ranked by % change 2018 - 2022 Parameter Overall level of service Knowledge of staff responding to enquiries 1% Parameter rating 2022 Excellent -4% How easy is it to identify the correct person to speak to in this area Helpfulness of staff Approachability Speed of response to enquiries Transparency of fee structure for all applications Excellent -5% -8% -8% -11% -12% Good Excellent Excellent Excellent Good Q46 We would now like to ask you about the Finance department and fees. How would you rate the finance department in terms of .Scale of 1 to 5, 1 Very poor, 5 Excellent. Bases on chart exclude “don’t know” and “not applicable” MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

PARALLEL APPLICATONS Parallel applications are those which are submitted simultaneously for GB and NI national GB and NI-DCP GB and Centralised (CAP) MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey CAUTION LOW BASE

SUMMARY: PARALLEL APPLICATIONS 2022: Parallel applications GB & NI National Excellent Both variations and new MAs applications rated as good or excellent by 100% of respondents DCP Good Variations rated as good or excellent by 63% of respondents; New MA applications rated by 75% of respondent as good or excellent Centralised Good Both variations and new MA applications rated as good or excellent by 50% of respondents MV006 · Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

INTERNATIONAL: JOINT REVIEWS The VMD currently offers the possibility of joint reviews with Canada and is developing similar arrangements with other global regulators. The VMD is also in regular contact with the regulatory agencies of USA, New Zealand, Australia and Canada and are interested in developing further opportunities MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

SUMMARY: INTERNATIONAL JOINT REVIEWS 2022 Joint reviews The most useful countries with which the VMD could develop a relationship would be Ireland and / or USA MV006 · Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

INTERNATIONAL: REPUTATION All who have interacted with another national authority within the last 2 years MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
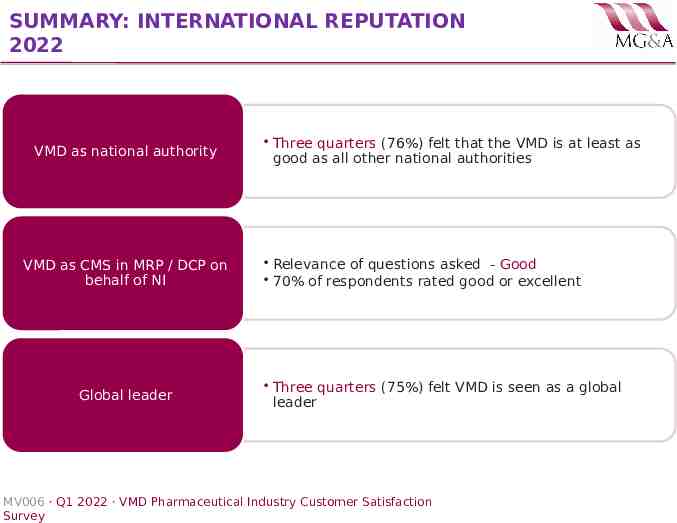
SUMMARY: INTERNATIONAL REPUTATION 2022 VMD as national authority VMD as CMS in MRP / DCP on behalf of NI Global leader Three quarters (76%) felt that the VMD is at least as good as all other national authorities Relevance of questions asked - Good 70% of respondents rated good or excellent Three quarters (75%) felt VMD is seen as a global leader MV006 · Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

OVERALL SATISFACTION WITH VMD MV006· Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey
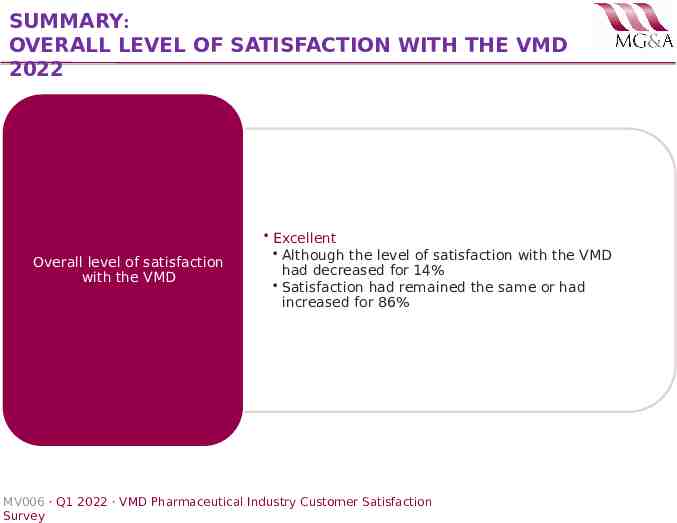
SUMMARY: OVERALL LEVEL OF SATISFACTION WITH THE VMD 2022 Overall level of satisfaction with the VMD Excellent Although the level of satisfaction with the VMD had decreased for 14% Satisfaction had remained the same or had increased for 86% MV006 · Q1 2022 · VMD Pharmaceutical Industry Customer Satisfaction Survey

Mo Gannon & Associates Ltd Henley-on-Thames Oxfordshire RG9 2HB T 44(0)1491 574937 M 44(0)7747 037231 [email protected] www.mogannonassociates.com






