Unit 4: Forensic Glass Analysis Introduction to Forensic Science
52 Slides2.35 MB

Unit 4: Forensic Glass Analysis Introduction to Forensic Science

Forensic Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 2

1. Composition of Glass Glass is a hard, brittle, amorphous material – Amorphous (without shape) because its atoms are arranged randomly – Due to its irregular atomic structure, it produces a variety of fracture patterns when broken – Brittle because it breaks easily if struck in the right direction Has numerous uses and thousands of compositions

1. Composition of Glass (continued) Made by melting the following ingredients at extremely high temperatures: – Sand also known as silica or silicon dioxide (SiO2), is the primary ingredient – Lime or calcium oxide (CaO) is added to prevent the glass from becoming soluble in water – Sodium oxide (Na2O) is added to reduce the melting point of silica or sand 4

1. Composition of Glass (continued) Three categories of substances found in all glass – Formers Makes up the bulk of the glass Examples: silicon dioxide (SiO2) in the form of sand, boron trioxide (B2O3), and phosphorus pentoxide (P2O5) – Fluxes Change formers’ melting points Examples: sodium carbonate (Na2CO3) and potassium carbonate (K2CO3) – Stabilizers Strengthen the glass and make it resistant to water Calcium carbonate (CaCO3) is the most frequently used 5
1. Composition of Glass (continued) The raw materials for making glass are all oxides (contain oxygen) – The composition of a sample can be expressed in percentage of different oxides – Example: the approximate composition of window or bottle glass is Silica (SiO2) – 73.6 % Soda (Na2O) – 16.0 % Lime (CaO) – 5.2 % Potash (K2O) – 0.6 % Magnesia (MgO) – 3.6 % Alumina (Al2O3) – 1.0 6
Video Clip – How Glass is Made 6:37 min https:// www.youtube.com/watch?v IjNusHQOhTM 5:04 min Plate Glass https:// www.youtube.com/watch?v PSurxsGQL90 7

Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 8

2. Types of Glass There are many different types of glass. We will discuss the following five types: A. Obsidian B. Soda Lime C. Leaded D. Tempered E. Laminated 9

2. Types of Glass A. Obsidian is a natural form of glass that is created by volcanoes B. Soda-lime glass – The most basic, common, inexpensive glass – also the easiest to make – Used for manufacturing windows and bottle glass 10

2. Types of Glass C. Borosilicate Glass - Contains boron oxide - heat-resistant glass: can be heated and cooled rapidly without breaking - Car headlights, Pyrex dishes, lab beakers and test tubes. 11

2. Types of Glass D. Leaded glass – Contains lead oxide which makes it denser – Sparkles as light passes through it (light waves are bent) – Used for manufacturing fine glassware and art glass (stained-glass windows) – Is commonly called crystal 12

2. Types of Glass E. Tempered glass – Stronger than ordinary glass – Strengthened by introducing stress through a cycle of rapid heating and cooling of its surface – When broken, this glass does not break into large shards, but fragments or breaks into small squares – Used in the side and rear windows of automobiles 13

2. Types of Glass F. Laminated glass – Constructed by bonding two ordinary sheets of glass together with a plastic film – Prevents shattering – Also used by automobile manufactures for windshields 14

Video Clip 5:34 min – Making Laminated Glass https:// www.youtube.com/watch?v fg3moEI9V5g 0:54 min – Laminated Glass Safety Test https://www.youtube.com/watch?v RVRAA4 VoAk0 5:34 min – Making Technical Glass https:// www.youtube.com/watch?v ixkZMyQ3Gac 15

Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 16

3. Comparing Glass Investigation/Analysis includes – Finding – Measuring – Comparing 17

3. Comparing Glass Individualized Characteristics – Only occurs if the suspect and crime scene fragments are fit together exactly, like a puzzle – This would require matching broken edges AND irregularities and striations on the broken surfaces – Most glass evidence is either too fragmented or too small to permit a comparison of this type So, what type of evidence is glass? 18

3. Comparing Glass (continued) Class Characteristics (Density and Refractive Index) – General composition of glass is a class characteristic – Trace elements in glass may prove to be distinctive and measureable characteristics, but still class – The physical properties of density and refractive index are used most successfully for characterizing glass particles, but only as a class characteristic 19

Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 20

3A. Methods of Comparison: Density Density is defined as the mass per unit volume. (D M/V) – Density is an intensive property of matter, meaning it remains the same regardless of sample size. – Density is considered a characteristic property of a substance and can be used as an aid in identification
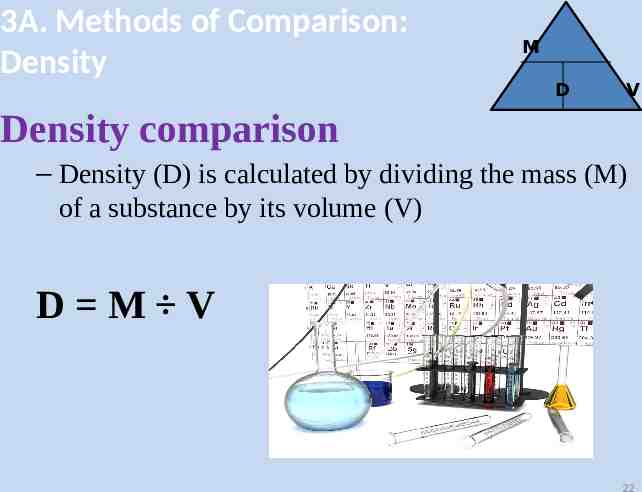
3A. Methods of Comparison: Density M D V Density comparison – Density (D) is calculated by dividing the mass (M) of a substance by its volume (V) D M V 22

3A. Density Density comparison (continued) Step 1: Find the sample’s volume by Archimedes’ Principal – – – – Fill a beaker half way, and put it on the scale Record the initial mass Gently lower the glass into the water attached to a string Record the final mass The density of water 23
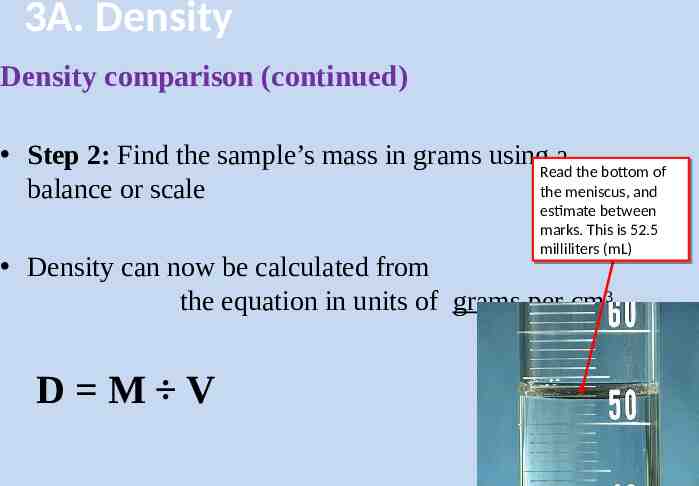
3A. Density Density comparison (continued) Step 2: Find the sample’s mass in grams using a Read Read the the bottom bottom of of the balance or scale the meniscus, meniscus, and and estimate estimate between between marks. marks. This This isis 52.5 52.5 milliliters milliliters (mL) (mL) Density can now be calculated from the equation in units of grams per cm3 D M V 24

Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 25

3B. Refractivity Let’s talk about light. What do you know? 26
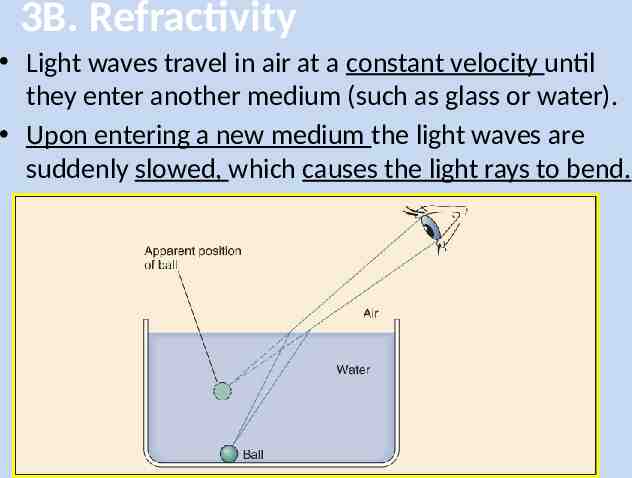
3B. Refractivity Light waves travel in air at a constant velocity until they enter another medium (such as glass or water). Upon entering a new medium the light waves are suddenly slowed, which causes the light rays to bend.

3B. Refractivity The bending of light waves because of a change in velocity is called refraction. The Refractive Index for a medium is a measure of how much light slows (or speeds up) upon entering that medium. For example: – At 25oC the refractive index of water is 1.333. – This means that light travels 1.333 times faster in a vacuum than it does in water.
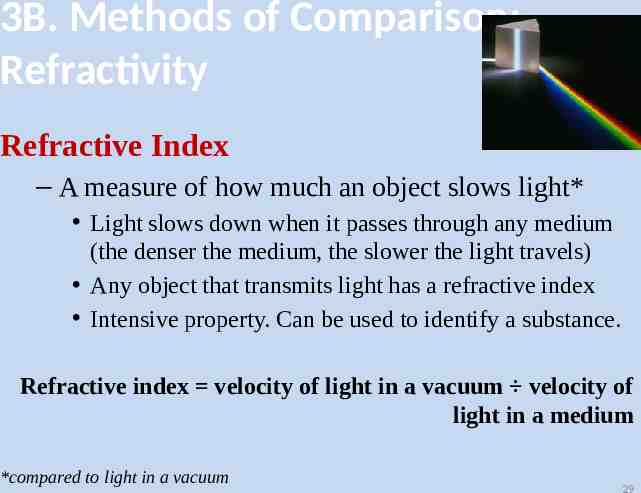
3B. Methods of Comparison: Refractivity Refractive Index – A measure of how much an object slows light* Light slows down when it passes through any medium (the denser the medium, the slower the light travels) Any object that transmits light has a refractive index Intensive property. Can be used to identify a substance. Refractive index velocity of light in a vacuum velocity of light in a medium *compared to light in a vacuum 29
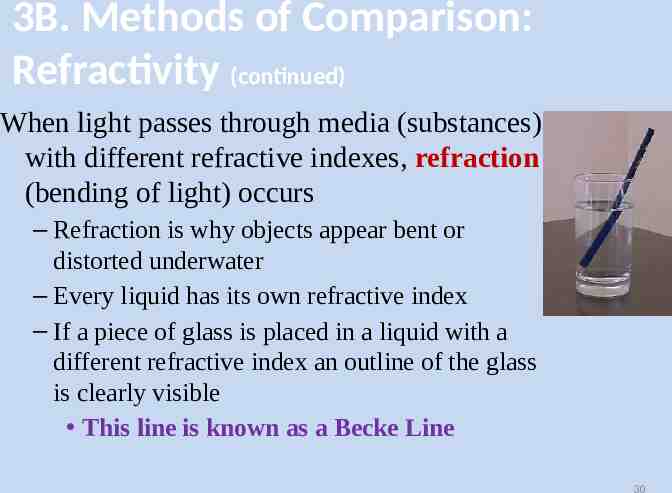
3B. Methods of Comparison: Refractivity (continued) When light passes through media (substances) with different refractive indexes, refraction (bending of light) occurs – Refraction is why objects appear bent or distorted underwater – Every liquid has its own refractive index – If a piece of glass is placed in a liquid with a different refractive index an outline of the glass is clearly visible This line is known as a Becke Line 30

3B. Methods of Comparison: Refractivity (continued) When light passes through a piece of glass placed in a liquid with the same refractive index: – The glass bends light at the same angle as the liquid – The Becke Lines disappear – The glass seems to disappear 31

Video Clip 4:14 Becke Line Demo https://www.youtube.com/watch?v qH1S83Bkttw 1:34 min Beck Line Test https://www.youtube.com/watch?v B6Gis-wloSI 5:04 The Becke Line Test https:// www.youtube.com/watch?v 1QA1EhsfTQY 32

Lab 4.2: Identifying Unknown Glass by Density and Refractive Index
Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 34

4. Glass Fracture Patterns Glass has a degree (amount) of elasticity (flexible-ness) – It breaks when its elastic limit is exceeded – The elasticity produces fractures when it is penetrated by a projectile (i.e. a bullet) 35

4. Glass Fracture Patterns When the elasticity of glass is exceeded, it fractures (breaks). There are several distinct types of glass fracture patterns: A. Radial B. Concentric C. Conchoidal 36

4. Glass Fracture Patterns (continued) A. Radial – Produced first – Form on the side of the glass opposite to where the impact originated (on the back) – Look like spider webs that spread outward from the impact hole – Always terminate (stop) at an existing fracture 37

A. Radial Fractures
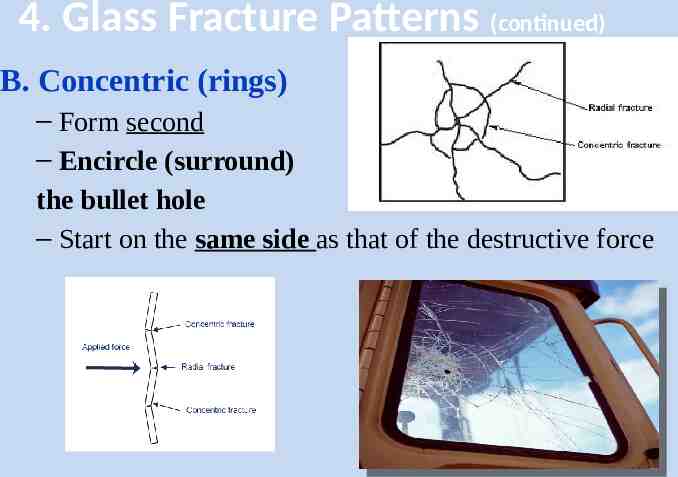
4. Glass Fracture Patterns (continued) B. Concentric (rings) – Form second – Encircle (surround) the bullet hole – Start on the same side as that of the destructive force 39
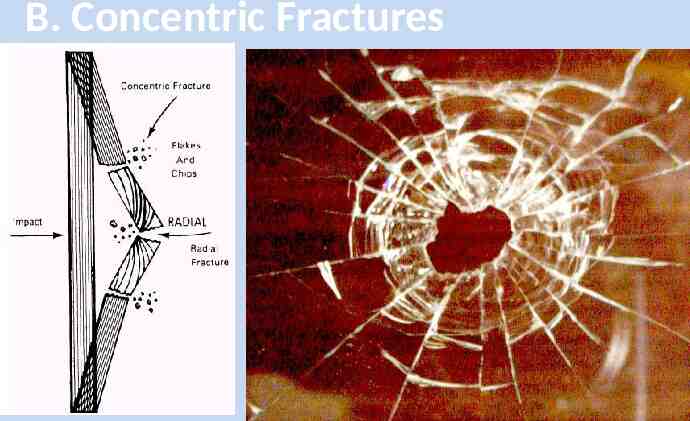
B. Concentric Fractures
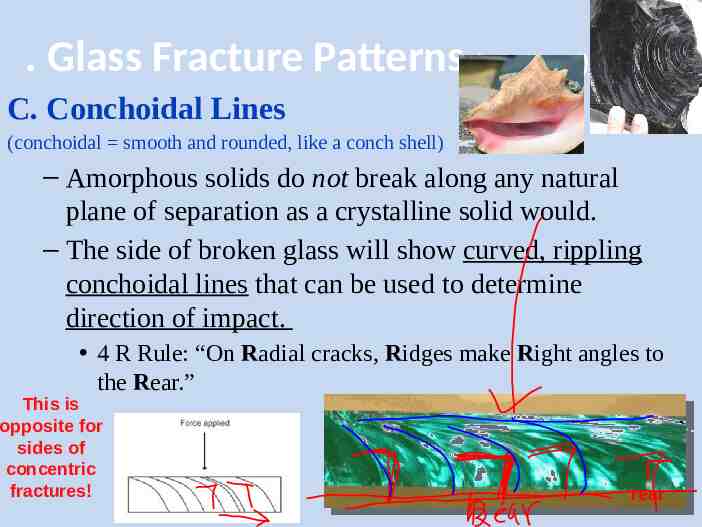
. Glass Fracture Patterns (continued) C. Conchoidal Lines (conchoidal smooth and rounded, like a conch shell) – Amorphous solids do not break along any natural plane of separation as a crystalline solid would. – The side of broken glass will show curved, rippling conchoidal lines that can be used to determine direction of impact. 4 R Rule: “On Radial cracks, Ridges make Right angles to the Rear.” This is opposite for sides of concentric fractures! rear

Video Clip 0:54 min – High Speed Glass Fracture https:// www.youtube.com/watch?v dwnBGnKSG2o 3:57 min – Tempered vs. Plate Glass Fracture https:// www.youtube.com/watch?v CiGH-W9rDbA 2:43 min – Bullet patterns in glass https:// www.youtube.com/watch?v QA1PG JhkWM 42
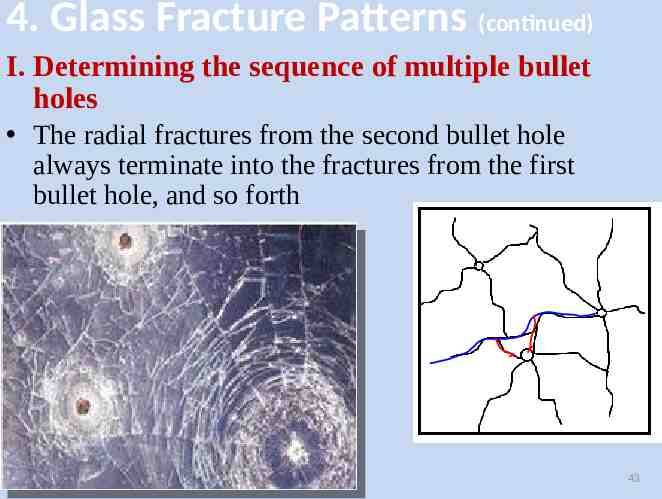
4. Glass Fracture Patterns (continued) I. Determining the sequence of multiple bullet holes The radial fractures from the second bullet hole always terminate into the fractures from the first bullet hole, and so forth 43
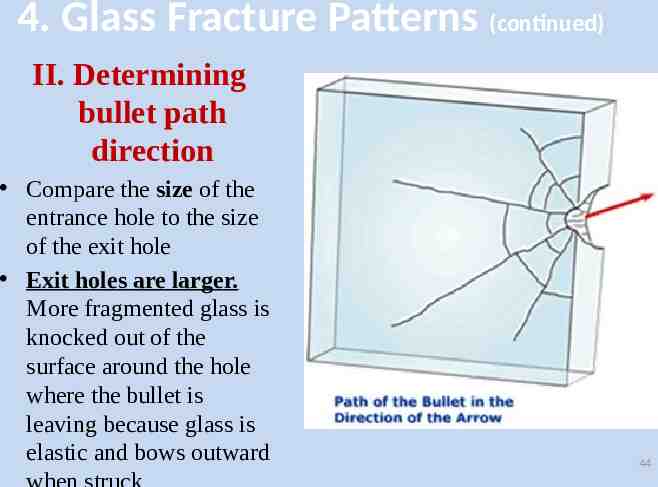
4. Glass Fracture Patterns (continued) II. Determining bullet path direction Compare the size of the entrance hole to the size of the exit hole Exit holes are larger. More fragmented glass is knocked out of the surface around the hole where the bullet is leaving because glass is elastic and bows outward 44

4. Glass Fracture Patterns (continued) II. Determining bullet path direction Entrance holes – The bullet makes a very small hole when it enters – The glass blows back in the direction of the impact because of its elasticity – The glass snaps back violently after being stressed and can blow shattered glass back several meters – Most of the shattered glass lands on the impacted side (front) of the glass, instead of by the exit hole Shot fired into Obama campaign office in Denver, CO (Oct. 2012) Source: telegraph.co.uk

Lab 4.1: Glass Fracture Patterns 46

Glass Analysis 1. Composition of Glass 2. Types of Glass 3. Comparing Glass A. Density B. Refractivity 4. Glass Fracture Patterns 5. Collecting Glass As Evidence 47

5. Collecting Glass as Evidence Avoid the loss or contamination of any evidence samples Identify and photograph all glass samples before moving them Collect the largest fragments Identify the outside and inside surfaces of any glass Indicate the relative position of multiple window panes in a diagram 48

5. Collecting Glass as Evidence (continued) Note any other trace evidence found on or embedded in the glass, such as skin, hair, blood, or fibers Package all of the collected materials properly in order to maintain the chain of custody Separate the glass by physical properties, such as size, color, and texture 49

5. Collecting Glass as Evidence (continued) Catalog the samples and keep them separated in order to avoid contamination between two different sources Separate the glass fragments from any other trace evidence (e.g., hair, blood, fibers) once in the lab Examine any clothing (or other objects that may have been used to break the glass) related to the crime scene for glass fragments and other trace evidence 50

Video Clips 6:26 min – Bullet Proof Glass Vandalism Test https://www.youtube.com/watch?v EibjrMy1 TtI 6:41 min – Bulletproof vests https:// www.youtube.com/watch?v eRabvfq8TdA 10:06 min – The Bulletproof Tailor https:// www.youtube.com/watch?v nQM6zLiSn1E 51

Resources Texas Education Agency, Forensic Certification Training, Sam Houston State University Forensic Science: Fundamentals & Investigation (1 st Edition), Anthony Bertino Forensic Science: From the Crime Scene to the Crime Lab (1 st Edition), Richard Saferstein ChemMatters, “More Than Meets The Eye” Brian Rohrig The Science Spot – Forensic Science – http://www.sciencespot.net/Pages/classforsci.html Investigator/Officer’s Personal Experience Corning Museum of Glass site – http://www.cmog.org/default.asp Federal Bureau of Investigation: Laboratory Services – Forensic Glass Comparison: Background Information Used in Data Interpretation http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/april2009/review – Introduction to Forensic Glass Examination http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/jan2005/standards/2005standards4.htm/ – Collection, Handling, and Identification of Glass http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/jan2005/standards/2005standards5.htm/ – Glass Density Determination http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/jan2005/standards/2005standards8.htm/ 52






