SARS-CoV-2 Testing Considerations Nimalie D. Stone, MD Long-term
18 Slides3.15 MB

SARS-CoV-2 Testing Considerations Nimalie D. Stone, MD Long-term Care Team Lead Kara M. Jacobs Slifka, MD Long-term Care Team Nursing Home COVID-19 Action Network Conversation Series For more information: www.cdc.gov/COVID19

Financial Disclosures No disclosures to report.

Learning topics Describe the different SARS-CoV-2 testing options and considerations for their use Apply the SARS-CoV-2 testing guidance for nursing home residents and healthcare personnel (HCP) Discuss factors that impact the interpretation of test results
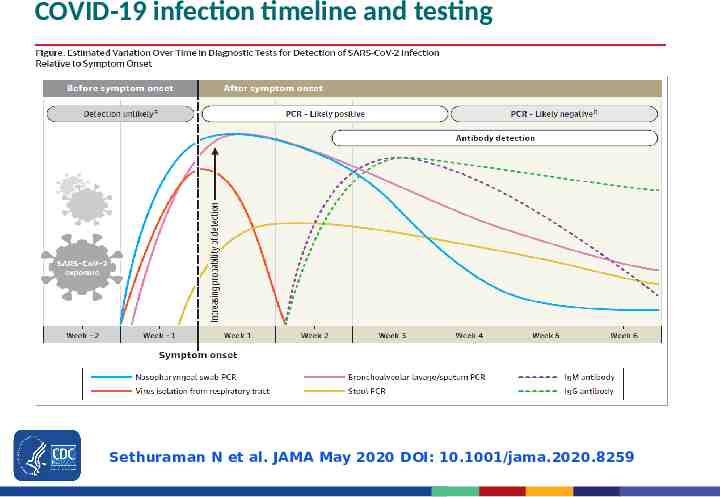
COVID-19 infection timeline and testing Sethuraman N et al. JAMA May 2020 DOI: 10.1001/jama.2020.8259
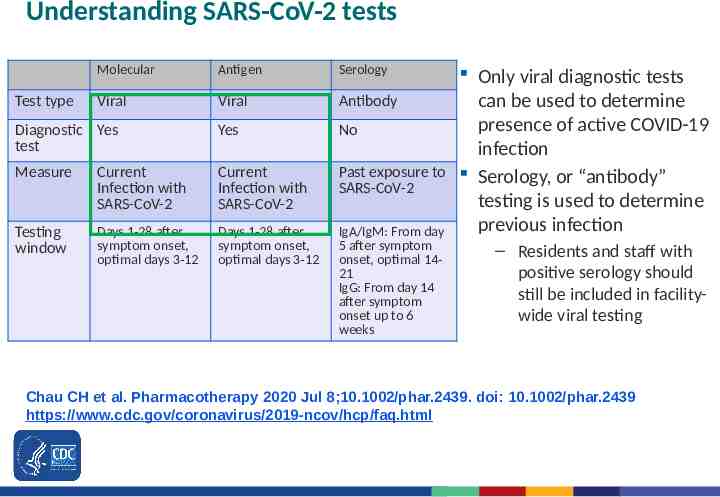
Understanding SARS-CoV-2 tests Molecular Antigen Serology Viral Viral Antibody Diagnostic Yes test Yes No Measure Current Infection with SARS-CoV-2 Current Infection with SARS-CoV-2 Past exposure to SARS-CoV-2 Testing window Days 1-28 after symptom onset, optimal days 3-12 Days 1-28 after symptom onset, optimal days 3-12 IgA/IgM: From day 5 after symptom onset, optimal 1421 IgG: From day 14 after symptom onset up to 6 weeks Test type Only viral diagnostic tests can be used to determine presence of active COVID-19 infection Serology, or “antibody” testing is used to determine previous infection – Residents and staff with positive serology should still be included in facilitywide viral testing Chau CH et al. Pharmacotherapy 2020 Jul 8;10.1002/phar.2439. doi: 10.1002/phar.2439 https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html
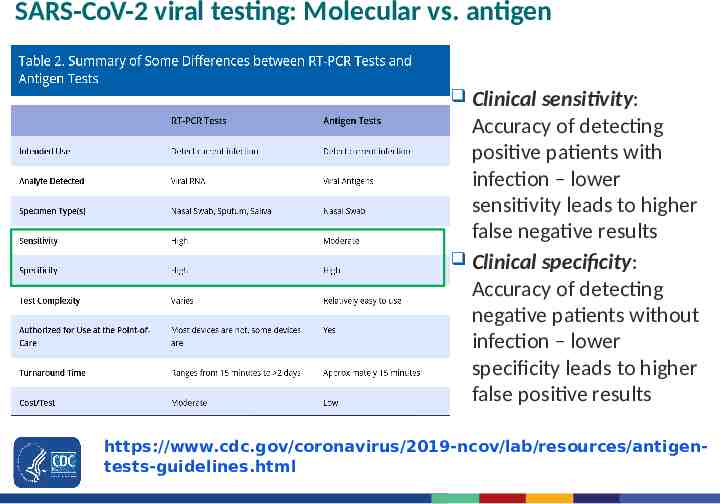
SARS-CoV-2 viral testing: Molecular vs. antigen Clinical sensitivity: Accuracy of detecting positive patients with infection – lower sensitivity leads to higher false negative results Clinical specificity: Accuracy of detecting negative patients without infection – lower specificity leads to higher false positive results https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigentests-guidelines.html
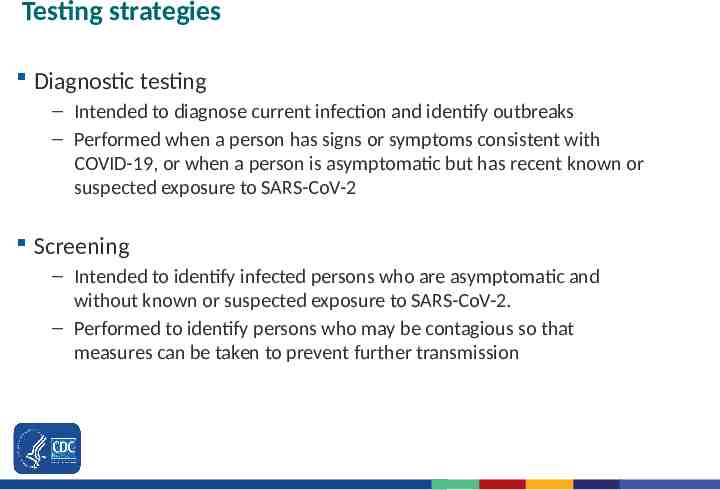
Testing strategies Diagnostic testing – Intended to diagnose current infection and identify outbreaks – Performed when a person has signs or symptoms consistent with COVID-19, or when a person is asymptomatic but has recent known or suspected exposure to SARS-CoV-2 Screening – Intended to identify infected persons who are asymptomatic and without known or suspected exposure to SARS-CoV-2. – Performed to identify persons who may be contagious so that measures can be taken to prevent further transmission

Current recommendations for testing in nursing homes Diagnostic testing: – Test any symptomatic residents and HCP immediately – Testing practices should aim for rapid turnaround times (e.g., less than 24 hours) in order to facilitate effective interventions Outbreak testing: – Triggered by a new SARS-CoV-2 infection in any HCP or any nursing home-onset SARS-CoV-2 infection in a resident Non-outbreak testing: – Baseline testing: Test all residents and staff once as part of reopening – Serial staff screening: test asymptomatic staff at frequency determined by county positivity (monthly, weekly, twice weekly) https://www.cms.gov/files/document/qso-20-38-nh.pdf https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homestesting.html

Outbreak testing in response to a new SARS-CoV-2 case Expand diagnostic testing for all residents and HCP – Initiate facility-wide testing as soon the first SARS-CoV-2 case is confirmed Perform repeat testing of all previously negative residents and HCP – Optimal outbreak testing occurs every 3 days during the first 14 days from the initial case identification; followed by testing every 7 days – Continue serial testing until no new positive cases are identified for a period of 14 days from the most recent positive result. – If testing capacity is limited, prioritize testing for residents with known exposure to a case, residents and HCP on affected units, and residents who leave and return to the facility https://www.cms.gov/files/document/qso-20-38-nh.pdf https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homestesting.html
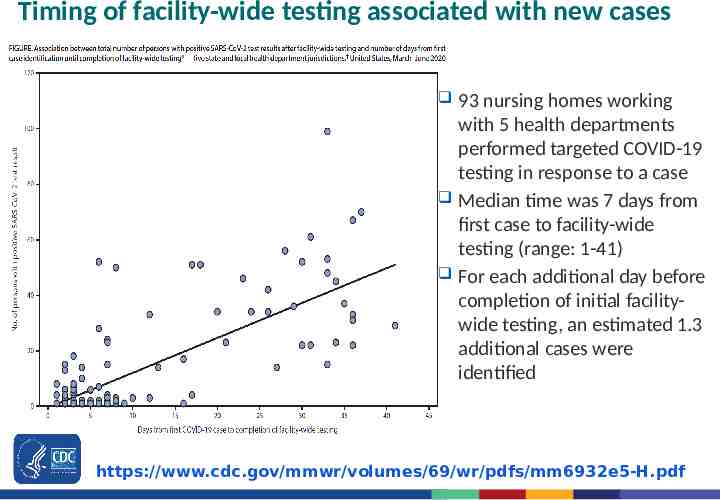
Timing of facility-wide testing associated with new cases 93 nursing homes working with 5 health departments performed targeted COVID-19 testing in response to a case Median time was 7 days from first case to facility-wide testing (range: 1-41) For each additional day before completion of initial facilitywide testing, an estimated 1.3 additional cases were identified https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6932e5-H.pdf

Considerations when implementing testing in nursing homes Managing residents and HCP clinically recovered from COVID-19 – If within 3 months of symptom onset of their most recent illness, no need to quarantine or retest for SARS-CoV-2 during outbreak response or staff screening – If testing positive for SARS-CoV-2 more than 3 months from recovery, should be considered infectious and placed in isolation or work exclusion – Retesting within first 3 months may be warranted for new symptoms consistent with COVID-19 if alternative etiologies for the illness cannot be identified Unclear benefit to regular screening tests for asymptomatic residents outside of outbreak response – Could result in false-positive results and lead to unnecessary testing – Consideration could be given to testing asymptomatic residents who frequently leave the facility of medical treatment, especially in communities with moderate to substantial SARS-CoV-2 transmission https://www.cdc.gov/coronavirus /2019-ncov/hcp/faq.html#Testing-in-Nursing-Homes https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Patients-with-
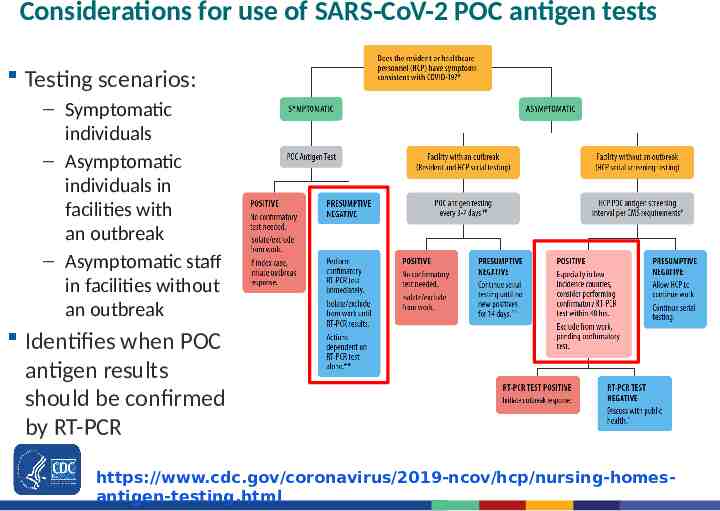
Considerations for use of SARS-CoV-2 POC antigen tests Testing scenarios: – Symptomatic individuals – Asymptomatic individuals in facilities with an outbreak – Asymptomatic staff in facilities without an outbreak Identifies when POC antigen results should be confirmed by RT-PCR https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homesantigen-testing.html

Factors that can impact interpretation of test results Quality of the specimen collection – Inadequate sampling or specimen mishandling – Running tests on specimens collected outside of the recommended time period recommended by manufacturer’s instructions for use Proper use of the testing platform – Trained personnel, proficient in sample handling with dedicated time – Space designated for running POC tests should be free of clutter, with regular surface cleaning/disinfection to prevent sample contamination – Quality controls should be used according to manufacturer’s instructions for use (e.g., new operators, new lots of test kits/reagents) Clinical presentation at the time of the test (e.g., symptoms) Prevalence of COVID-19 infections in the center and community https://www.youtube.com/watch?v 8oCRqlY1kJw https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafetyguidelines.html#decentralized

Responding to POC antigen results While awaiting confirmatory test results for potential false-negative or false-positive antigen test results, maintain IPC measures (e.g., HCP work exclusion, resident placement in Transmission-Based Precautions) – Select a confirmatory test with high sensitivity (e.g., RT-PCR) – Perform confirmatory test within 2 days of initial result – Additional testing of asymptomatic residents or other close contacts can be delayed until results of confirmatory testing are available, unless additional symptomatic individuals are identified – Only move residents with confirmed infection to a dedicated COVID-19 unit Confirmatory RT-PCR testing after a positive antigen test result is not recommended when the person being tested is symptomatic or had recent exposure to a SARS-CoV-2 case (e.g. during an outbreak) https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Testin g-in-Nursing-Homes

Limitations to SARS-CoV-2 testing A single negative test may not rule out COVID-19 infection in asymptomatic individuals – A person can be incubating SARS-CoV-2 for up to 14 days before manifesting clinical illness or having detectable virus – Testing immediately before or after admission cannot be used to remove a resident from 14-day quarantine Clinicians must consider the likelihood of COVID-19 infection as part of interpreting test results – A negative test in someone with exposure and symptoms consistent with COVID-19 infection should be verified – A positive test in an asymptomatic person, in a community with low prevalence of COVID-19 infection should be verified Testing alone cannot prevent the spread of SARS-CoV-2 – Facilities must remain committed to all infection prevention strategies to protect residents and staff

CDC Testing Guidance and FAQs https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homestesting.html https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-healthcare-p ersonnel.html

COVID-19 Resources for Nursing Homes Infection Control Guidance SARS-CoV-2 Testing Guidance Assessment tools Training resources https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursinghome-long-term-care.html

For more information, contact CDC 1-800-CDC-INFO (232-4636) TTY: 1-888-232-6348 www.cdc.gov Thank you! The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.






