QuantiFERON®-TB Gold Test A 100 Year Update for the Diagnosis of
20 Slides638.00 KB

QuantiFERON -TB Gold Test A 100 Year Update for the Diagnosis of Tuberculosis Infection Alfred Lardizabal, MD New Jersey Medical School Global Tuberculosis Institute

Introduction In the U.S., the effort to control tuberculosis, its transmission, and ultimately, its eradication has been fought along two important fronts The first front is to detect and treat people with infectious tuberculosis The second front is to detect high risk asymptomatic people who have latent TB infection and prevent the development of active disease
Introduction The Institute of Medicine and the CDC recognizes the importance of developing accurate diagnostic tests for TB infection to hasten the process of TB elimination A sensitive test would accurately identify people with infection, whether latent or active (maximize true positive results) A specific test would accurately identify people who are uninfected (maximize true negative)
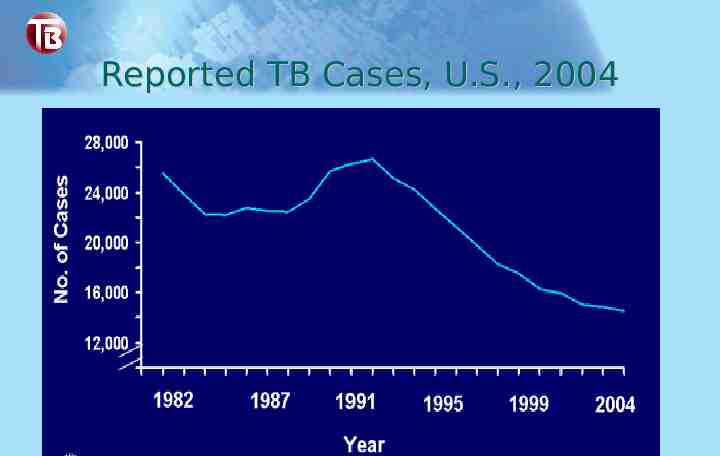
Reported TB Cases, U.S., 2004
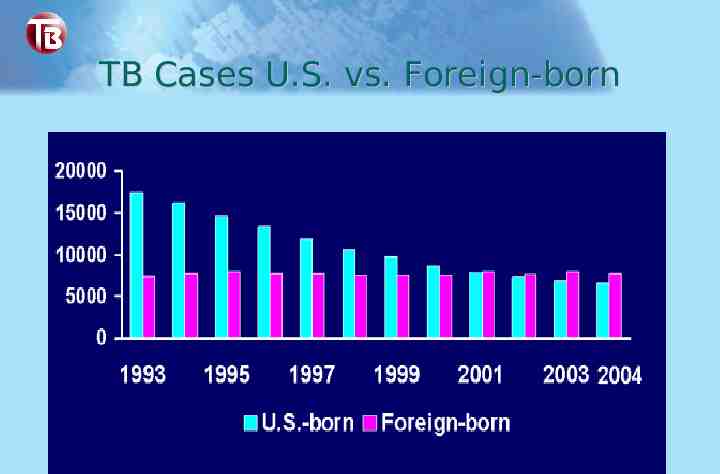
TB Cases U.S. vs. Foreign-born

Tuberculin skin test Until recently, the standard method for the immunologic diagnosis of M. tuberculosis infection has been limited to the tuberculin skin test Has been used to detect both LTBI and active tuberculosis Concerns abound about its lack of sensitivity and specificity resulting in false positive and false negative results

TST - Historical perspective Tuberculin was developed a decade after the discovery of the tubercle bacillus as the cause of TB The original preparation (old tuberculin) was obtained from heat sterilized cultures of tubercle bacilli Initially touted as therapeutic, which was found to be disappointing, its use eventually led to the discovery of its diagnostic value

TST - Historical perspective Old tuberculin was an unrefined product contributing to its lack of sensitivity in the diagnosis of infection with M. tuberculosis Refinements to the OT preparation led to the development of PPD, still used in present day Mantoux skin testing

TST: False negatives / False positives False negatives False positives Technical factors – Application – Reading – Improper storage of PPD Infection with nontuberculous mycobacteria Biological factors – Poor nutrition – Infection – Immunosuppressive drugs – Malignancy – Age – Stress BCG vaccination
What is Quanti-FERON -TB Gold Blood assay for M. tuberculosis Interferon γ release assay In vitro test using whole blood specimen for the diagnosis of TB infection, whether latent or active Does not distinguish between latent TB infection or TB disease
Quanti-FERON -TB Gold – Scientific Basis Individuals infected with M. tuberculosis complex organisms have lymphocytes in their blood that recognize mycobacterial antigens This recognition process involves the generation of interferon-γ, a specific cytokine for cell mediated immune response The detection and subsequent quantification of IFN-γ is the basis of this test The test uses synthetic peptide antigens (ESAT-6, CFP10) that simulate mycobacterial proteins to generate the immune response
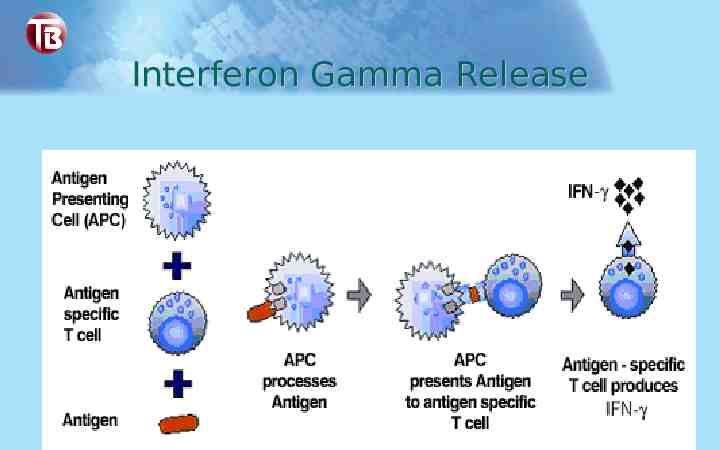
Interferon Gamma Release
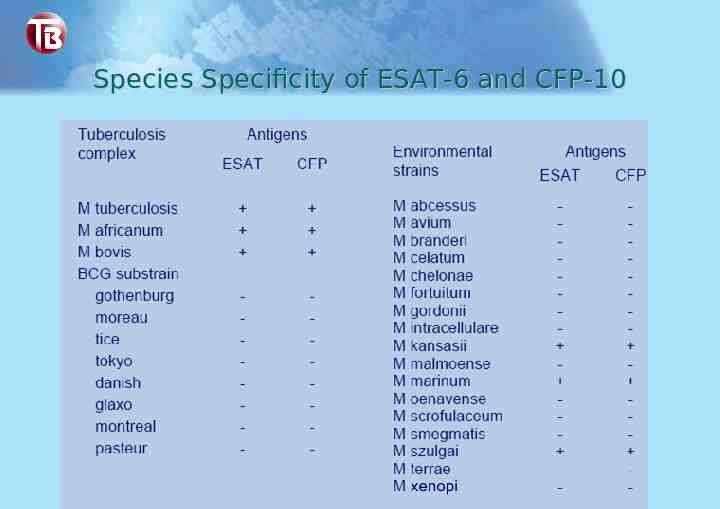
Species Specificity of ESAT-6 and CFP-10
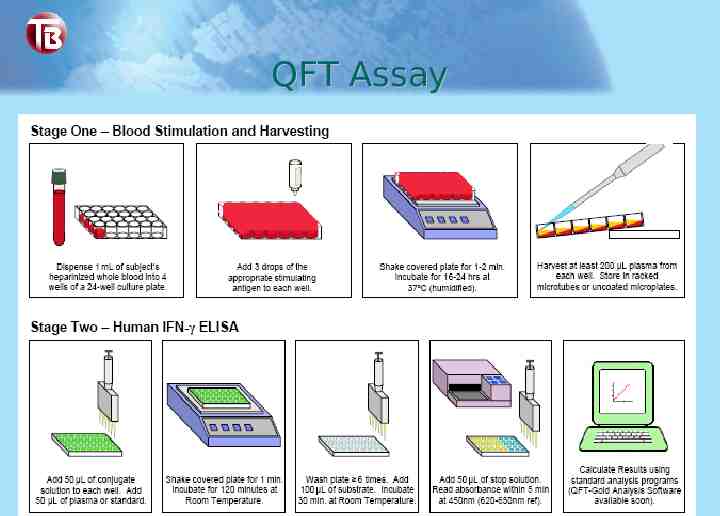
QFT Assay
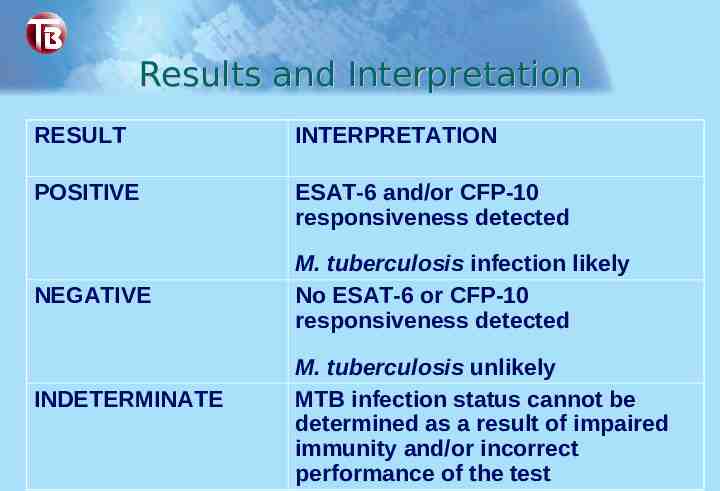
Results and Interpretation RESULT INTERPRETATION POSITIVE ESAT-6 and/or CFP-10 responsiveness detected NEGATIVE INDETERMINATE M. tuberculosis infection likely No ESAT-6 or CFP-10 responsiveness detected M. tuberculosis unlikely MTB infection status cannot be determined as a result of impaired immunity and/or incorrect performance of the test

Specificity Estimates 216 healthy individuals, no identified risk for TB infection, all BCG ( ) – Specificity 98% (213/216 QFT negative) Mori, et al. AJRCCM 2004;170:59-64 532 with no identified risk for TB infection among Navy recruits – Specificity 99.8% (531/532 QFT negative) CDC; publication in preparation 99 healthy individuals with no identified risk for TB infection, all BCG ( ) – Specificity 96% (95/99 QFT negative) Kang, et al. JAMA 2005;293:2756-2761
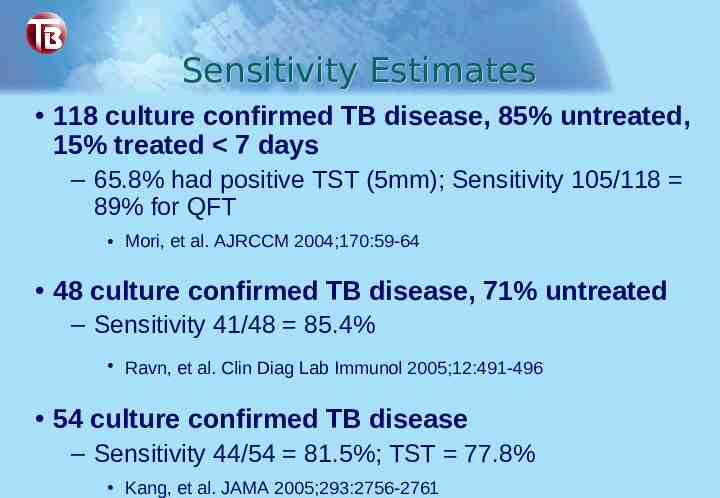
Sensitivity Estimates 118 culture confirmed TB disease, 85% untreated, 15% treated 7 days – 65.8% had positive TST (5mm); Sensitivity 105/118 89% for QFT Mori, et al. AJRCCM 2004;170:59-64 48 culture confirmed TB disease, 71% untreated – Sensitivity 41/48 85.4% Ravn, et al. Clin Diag Lab Immunol 2005;12:491-496 54 culture confirmed TB disease – Sensitivity 44/54 81.5%; TST 77.8% Kang, et al. JAMA 2005;293:2756-2761
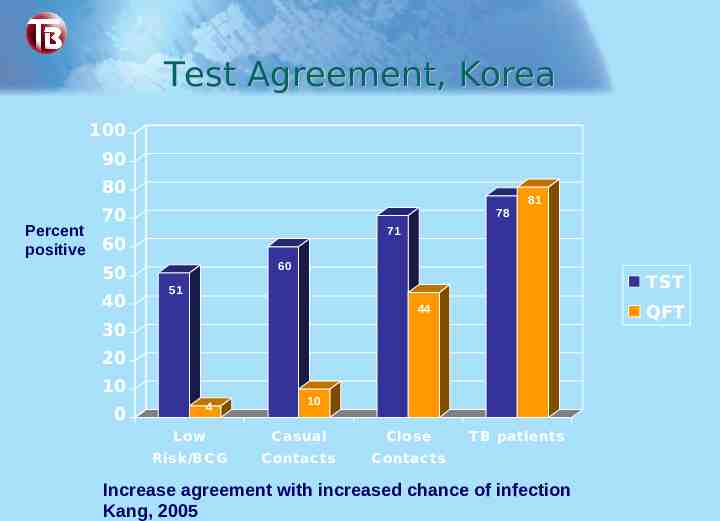
Test Agreement, Korea 100 90 80 Percent positive 70 78 71 60 60 50 40 81 TST 51 QFT 44 30 20 10 4 0 10 L ow C asual C lose Risk/BC G C ontac ts C ontac ts T B patients Increase agreement with increased chance of infection Kang, 2005
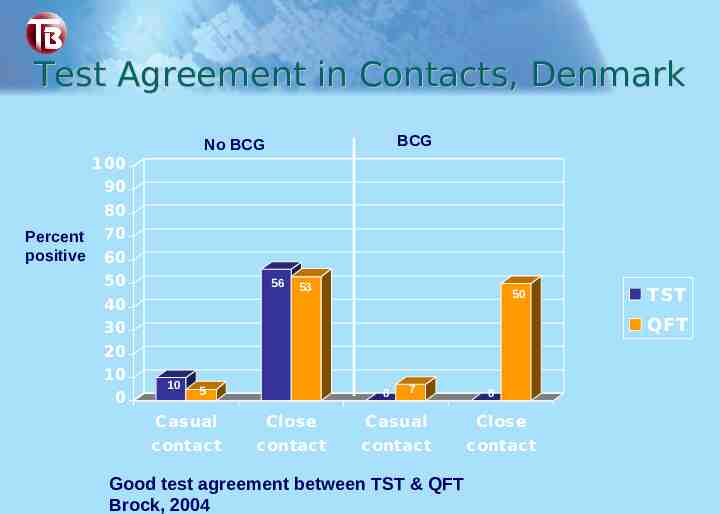
Test Agreement in Contacts, Denmark BCG No BCG 100 90 Percent positive 80 70 60 50 56 53 50 40 30 20 10 0 TST QFT 10 5 0 7 0 Casual Close Casual Close c ontac t c ontac t c ontac t c ontac t Good test agreement between TST & QFT Brock, 2004

QFT and TST QFT TST in vitro test in vivo test Specific antigens Less specific PPD No boosting Boosting 1 patient visit 2 patient visits Lab variability Inter-reader variability Results possible in 1 day Results in 2-3 days Requires phlebotomy No phlebotomy required Includes control No control