Premarket Review Performance Goals MDUFMA Stakeholder Meeting
44 Slides8.97 MB

Premarket Review Performance Goals MDUFMA Stakeholder Meeting November 18, 2004 Donna-Bea Tillman Director, Office of Device Evaluation Don St. Pierre Deputy Director, Office of In Vitro Diagnostic Device Evaluation and Safety

MDUFMA’s Promise FDA will turn dollars into shorter review times.

MDUFMA’s Challenge Meeting the MDUFMA goals while maintaining our commitment to good science.

How are we making this happen?

New Vision of Pre-Market Review Office of In Vitro Diagnostic Device Evaluation and Safety Office of Device Evaluation Office of Science & Engineering Laboratories Office of Surveillance & Biometrics Office of Communication, Education, and Radiation Programs Office of Compliance Office of Management Operations

What are we doing to foster this vision? Shared hires eRoom eConsults Shared agreement on timelines . - Shared commitment to the goals

How are we spending your money?

Hiring!
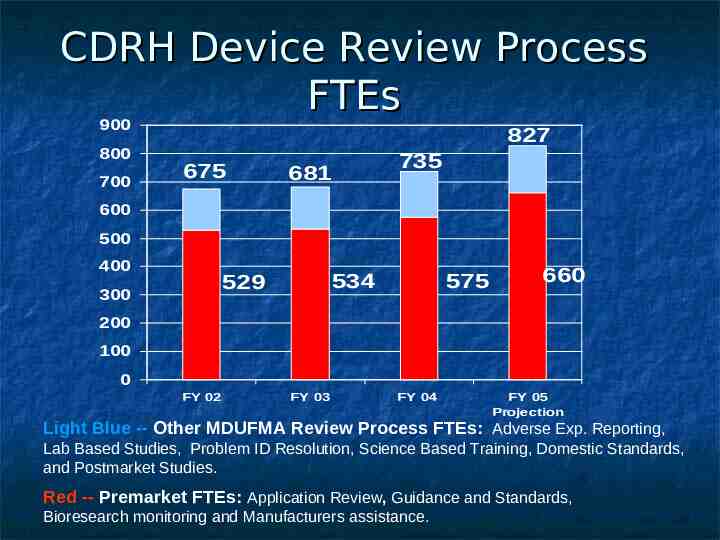
CDRH Device Review Process FTEs 900 800 700 827 675 735 681 600 500 400 534 529 300 575 660 200 100 0 FY 02 FY 03 FY 04 FY 05 Projection Light Blue -- Other MDUFMA Review Process FTEs: Adverse Exp. Reporting, Lab Based Studies, Problem ID Resolution, Science Based Training, Domestic Standards, and Postmarket Studies. Red -- Premarket FTEs: Application Review, Guidance and Standards, Bioresearch monitoring and Manufacturers assistance.
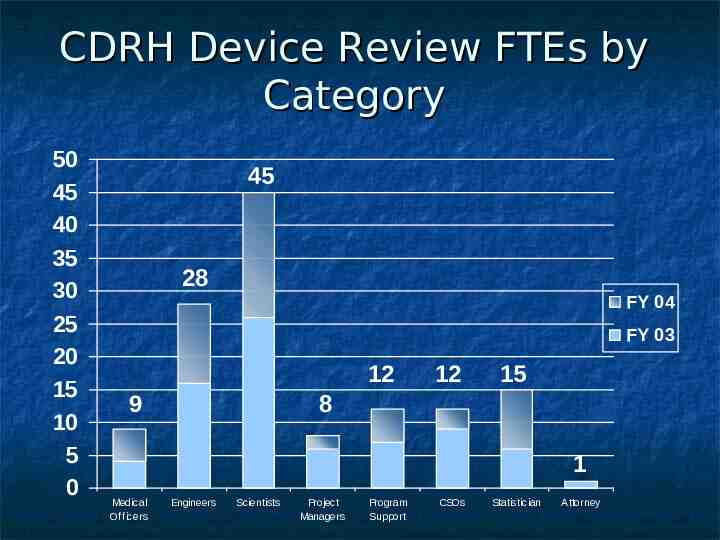
CDRH Device Review FTEs by Category 50 45 40 35 30 25 20 15 10 5 0 45 28 FY 04 FY 03 12 9 12 15 8 1 Medical Officers Engineers Scientists Project Managers Program Support CSOs Statistician Attorney

Medical Device Fellowship Program (FY04) Physicians* - 15 Engineers* - 42 Visiting Scholar – senior level clinicians, surgeons Fellow - physician during fellowship training Resident – physician during residency training Visiting Scholar – senior level engineer Co-op students Interns Physicists* - 2 Scientists* - 5 *includes students

Training! All those new hires need to be trained The good news: training for new recruits improved their proficiency The bad news: training for new recruits took a lot of resources and is not complete. We also need to maintain the skills of existing staff
Improved IT infrastructure! Current IT needs Tracking Reviewing Collaborating Managing correspondence Archiving - Image And maybe one day . Electronic Review?

Just the Numbers For complete report, please see: http://www.fda.gov/cdrh/mdufma/presentations/102004-kahan.ppt
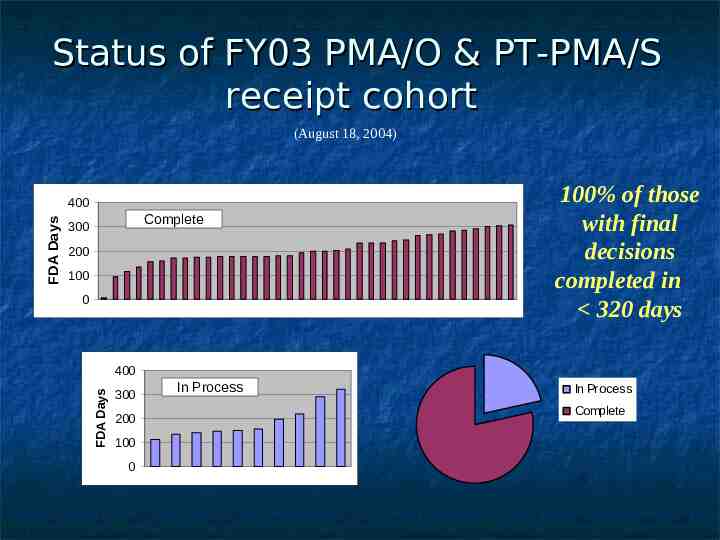
Status of FY03 PMA/O & PT-PMA/S receipt cohort (August 18, 2004) Complete 300 200 100 0 100% of those with final decisions completed in 320 days 400 FDA Days FDA Days 400 300 200 100 0 In Process In Process Complete
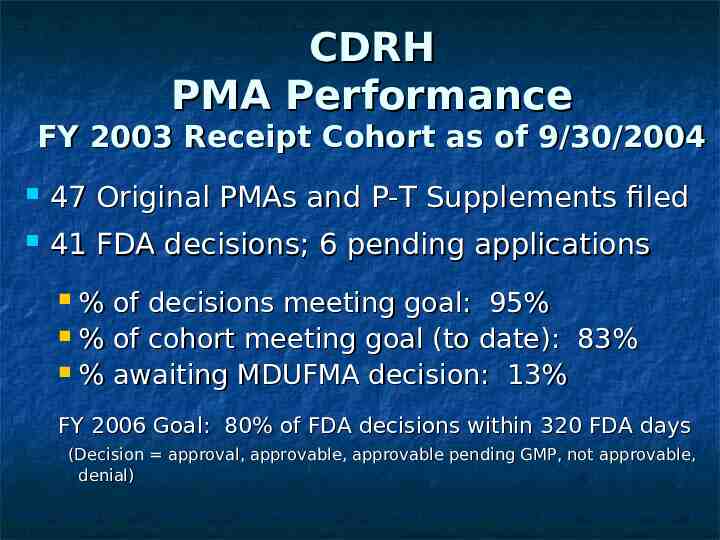
CDRH PMA Performance FY 2003 Receipt Cohort as of 9/30/2004 47 Original PMAs and P-T Supplements filed 41 FDA decisions; 6 pending applications % of decisions meeting goal: 95% % of cohort meeting goal (to date): 83% % awaiting MDUFMA decision: 13% FY 2006 Goal: 80% of FDA decisions within 320 FDA days (Decision approval, approvable, approvable pending GMP, not approvable, denial)

CDRH PMA Performance FY 2003 Receipt Cohort as of 9/30/2004 25 1st action major deficiency letter % of actions meeting goal: 84% FY 2005 Goal: 75% within 150 FDA days 22 “all other” 1st actions % actions meeting goal: 96% FY 2005 Goal: 75% 180 FDA days FY 2003 first action cohort is closed
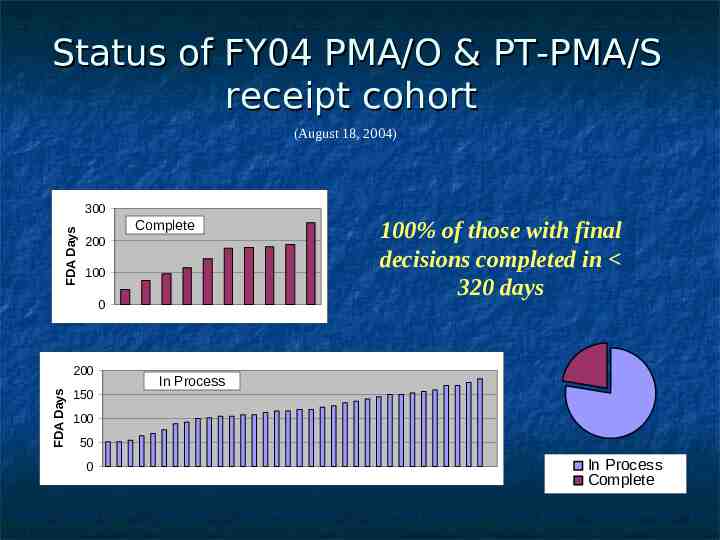
Status of FY04 PMA/O & PT-PMA/S receipt cohort (August 18, 2004) FDA Days 300 Complete 200 100 0 FDA Days 200 150 100% of those with final decisions completed in 320 days In Process 100 50 0 In Process Complete
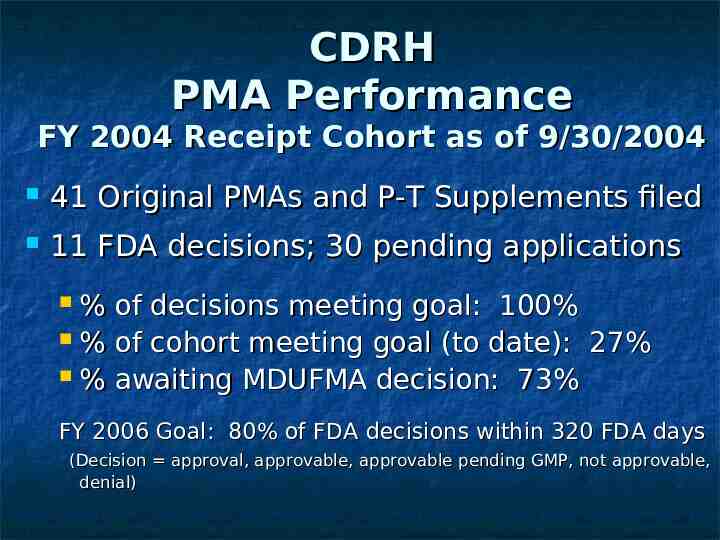
CDRH PMA Performance FY 2004 Receipt Cohort as of 9/30/2004 41 Original PMAs and P-T Supplements filed 11 FDA decisions; 30 pending applications % of decisions meeting goal: 100% % of cohort meeting goal (to date): 27% % awaiting MDUFMA decision: 73% FY 2006 Goal: 80% of FDA decisions within 320 FDA days (Decision approval, approvable, approvable pending GMP, not approvable, denial)

CDRH PMA Performance FY 2004 Receipt Cohort as of 9/30/2004 20 1st action major deficiency letter % of actions meeting goal: 85% FY 2005 Goal: 75% within 150 FDA days 12 “all other” 1st actions % actions meeting goal: 100% FY 2005 Goal: 75% 180 FDA days 9 with first action pending (22% of cohort)

Improving PMA performance Goal: Shorten both cycle and total decision times which will improve predictability and decrease time-tomarket.
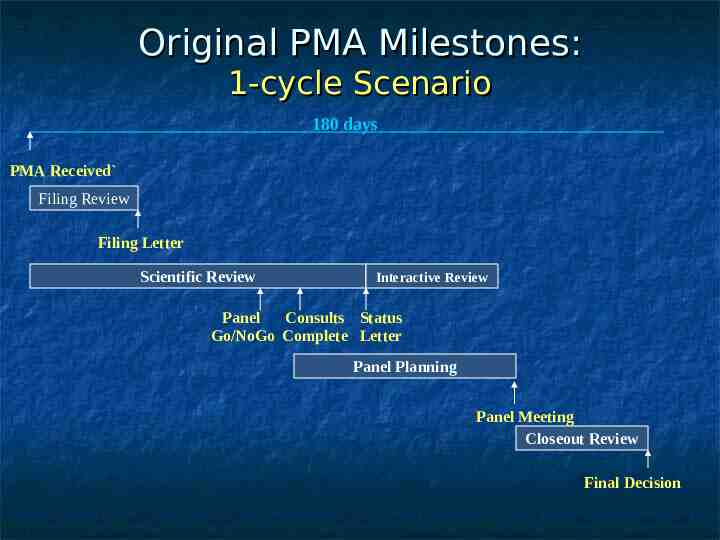
Original PMA Milestones: 1-cycle Scenario 180 days PMA Received Filing Review Filing Letter Scientific Review Interactive Review Panel Consults Status Go/NoGo Complete Letter Panel Planning Panel Meeting Closeout Review Final Decision
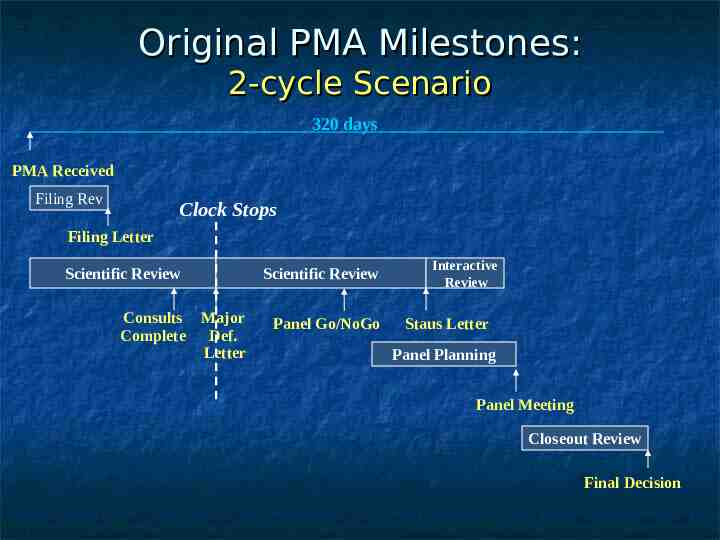
Original PMA Milestones: 2-cycle Scenario 320 days PMA Received Filing Rev Clock Stops Filing Letter Scientific Review Consults Major Complete Def. Letter Scientific Review Panel Go/NoGo Interactive Review Staus Letter Panel Planning Panel Meeting Closeout Review Final Decision

510k Goals
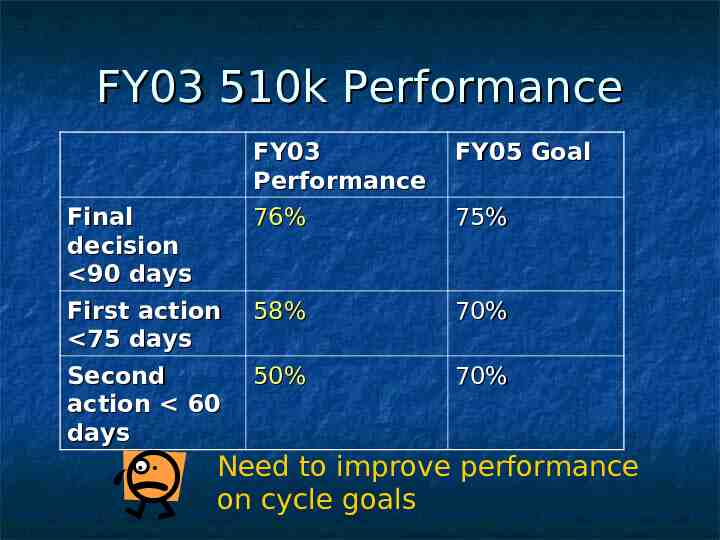
FY03 510k Performance Final decision 90 days First action 75 days Second action 60 days FY03 Performance 76% FY05 Goal 58% 70% 50% 70% 75% Need to improve performance on cycle goals

Meeting the MDUFMA goals: Review Process for Traditional 510ks Scenario #1 FDA Initial Review Interactive Review Final Decision ( 90 total days) Preliminary determination that no significant additional info needed

Meeting the MDUFMA goals: Review Process for Traditional 510ks Scenario #2 FDA Initial Review Hold Determination that significant additional info needed Interactive Review Preliminary determination that no significant additional info needed Final Decision ( 90 total days)

Meeting the MDUFMA goals: Review Process for Traditional 510ks Scenario #3 FDA Initial Review Hold Review Determination that significant additional info needed Hold Review Determination that significant additional info needed Final Decision ( 90 total days)
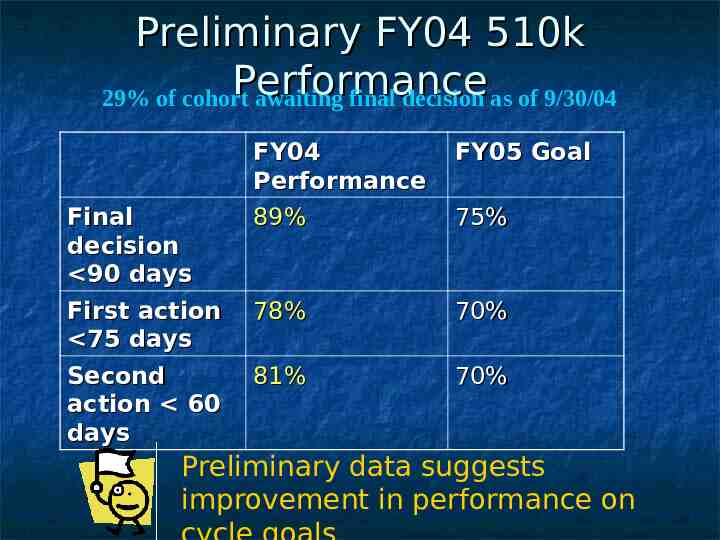
Preliminary FY04 510k Performance 29% of cohort awaiting final decision as of 9/30/04 Final decision 90 days First action 75 days Second action 60 days FY04 Performance 89% FY05 Goal 78% 70% 81% 70% 75% Preliminary data suggests improvement in performance on
What about the Science?

Program to Assess Quality of Premarket Reviews Focuses on selected cross-cutting areas (e.g., biocompatibility) Retrospective (post-decision) peer assessments of sampled reviews Quality assessment teams with defined criteria Pilot underway

Where have we been? We’ve made progress in meeting the goals, but it has come at a price.

The price of early success Our staff are working at the limits of their abilities “Luxuries” such as training, standards, and guidance development have been neglected

Where are we now? We’ll continue to improve performance, but there are additional challenges ahead.

The number of combination products is growing Coronary Stent Pacing Lead necessitating new kinds of technical expertise and new regulatory paradigms. Bone graft
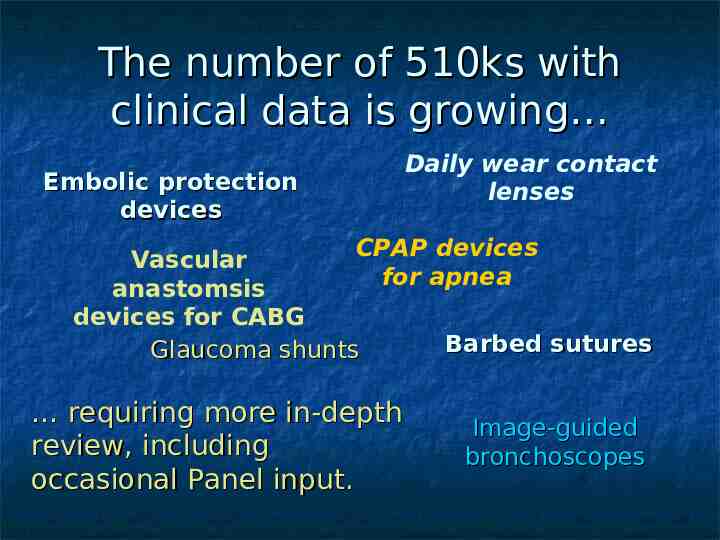
The number of 510ks with clinical data is growing Embolic protection devices Daily wear contact lenses CPAP devices Vascular for apnea anastomsis devices for CABG Barbed sutures Glaucoma shunts requiring more in-depth review, including occasional Panel input. Image-guided bronchoscopes

The number of expedited submissions is growing . 14 expedited PMAs in FYO4 vs. 3 expedited PMAs in FYO3 shortening timeframes and bringing increasingly complex scientific questions.

Where are we going? The best is yet to come!

Strategies for Meeting Beating the MDUFMA Goals Systematic management of timeframes Improvements to IT infrastructure Well-trained staff More guidance documents Open communication
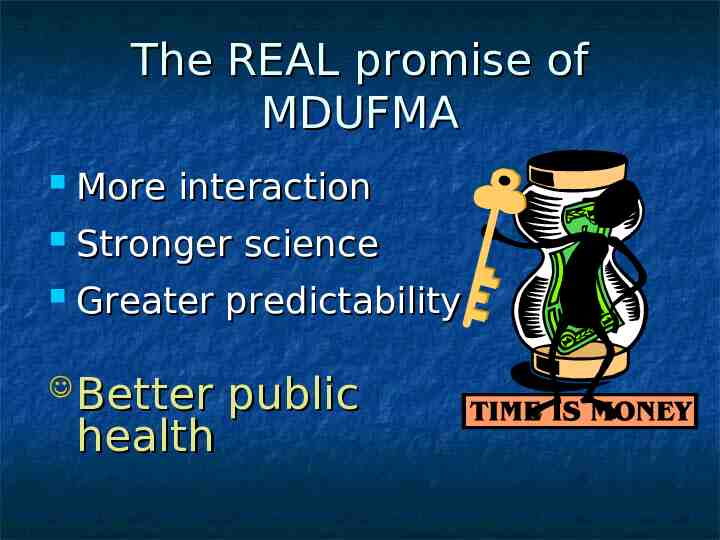
The REAL promise of MDUFMA More interaction Stronger science Greater predictability Better health public

and a continuing commitment to Least Burdensome Principles

Office of In Vitro Diagnostic Device Evaluation and Safety (OIVD) Turbo 510(k) Pilot Pre-IDEs Public/information sharing Webpage Re-design in process Number/interactions increasing Decision Summaries Software/program development Based on your feedback Internal training (challenging the status quo) Stressing TPLC, Communication, Collaboration

Office of In Vitro Diagnostic Device Evaluation and Safety (OIVD) Also, Proactive in: Bioterrorism activities to improve development and access to rapid diagnostics CLIA improvements (including development of waiver criteria, program development) Pharmacogenomics

Questions? MDUFMA