Neurobiology and aetiology Major depressive disorder 1
42 Slides2.36 MB

Neurobiology and aetiology Major depressive disorder 1

Introduction to neuroanatomy 2
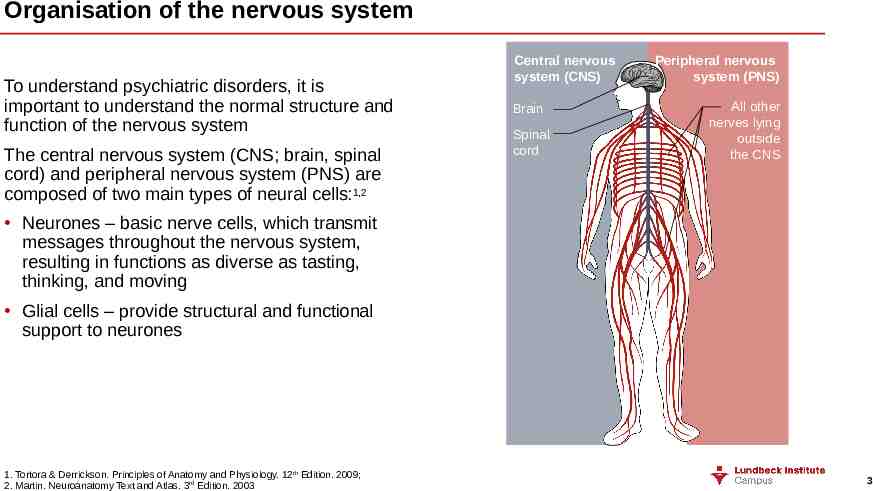
Organisation of the nervous system To understand psychiatric disorders, it is important to understand the normal structure and function of the nervous system The central nervous system (CNS; brain, spinal cord) and peripheral nervous system (PNS) are composed of two main types of neural cells:1,2 Central nervous system (CNS) Brain Spinal cord Peripheral nervous system (PNS) All other nerves lying outside the CNS Neurones – basic nerve cells, which transmit messages throughout the nervous system, resulting in functions as diverse as tasting, thinking, and moving Glial cells – provide structural and functional support to neurones 1. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009; 2. Martin. Neuroanatomy Text and Atlas. 3rd Edition. 2003 3
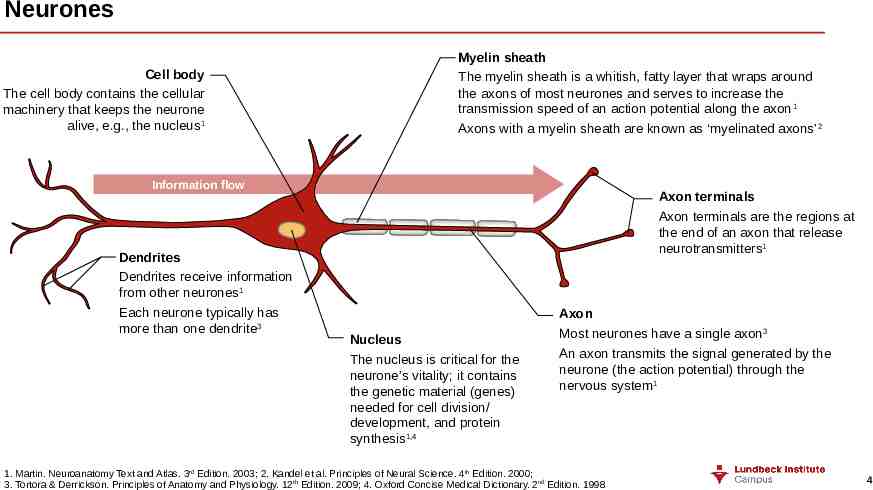
Neurones Cell body The cell body contains the cellular machinery that keeps the neurone alive, e.g., the nucleus 1 Myelin sheath The myelin sheath is a whitish, fatty layer that wraps around the axons of most neurones and serves to increase the transmission speed of an action potential along the axon 1 Axons with a myelin sheath are known as ‘myelinated axons’ 2 Information flow Dendrites Dendrites receive information from other neurones1 Each neurone typically has more than one dendrite3 Axon terminals Axon terminals are the regions at the end of an axon that release neurotransmitters1 Nucleus The nucleus is critical for the neurone’s vitality; it contains the genetic material (genes) needed for cell division/ development, and protein synthesis1,4 Axon Most neurones have a single axon3 An axon transmits the signal generated by the neurone (the action potential) through the nervous system1 1. Martin. Neuroanatomy Text and Atlas. 3rd Edition. 2003; 2. Kandel et al. Principles of Neural Science. 4th Edition. 2000; 3. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009; 4. Oxford Concise Medical Dictionary. 2nd Edition. 1998 4
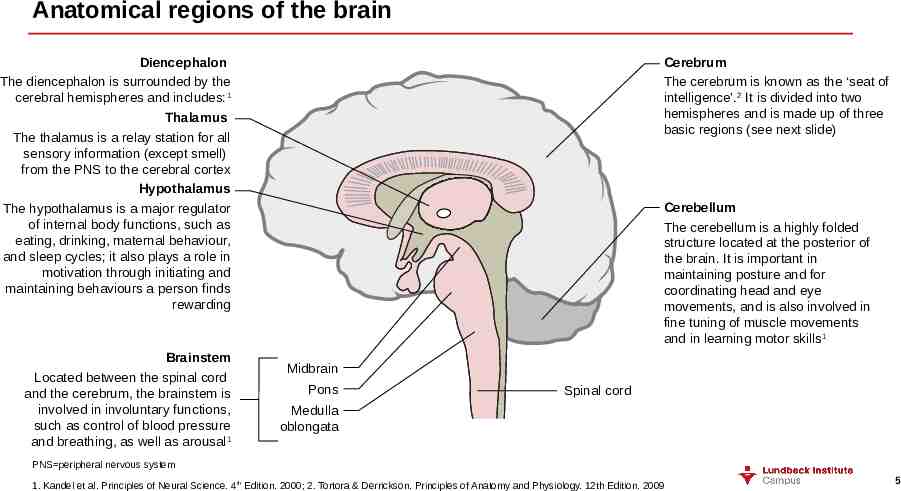
Anatomical regions of the brain Diencephalon The diencephalon is surrounded by the cerebral hemispheres and includes: 1 Thalamus The thalamus is a relay station for all sensory information (except smell) from the PNS to the cerebral cortex Hypothalamus The hypothalamus is a major regulator of internal body functions, such as eating, drinking, maternal behaviour, and sleep cycles; it also plays a role in motivation through initiating and maintaining behaviours a person finds rewarding Brainstem Located between the spinal cord and the cerebrum, the brainstem is involved in involuntary functions, such as control of blood pressure and breathing, as well as arousal 1 Cerebrum The cerebrum is known as the ‘seat of intelligence’.2 It is divided into two hemispheres and is made up of three basic regions (see next slide) Cerebellum The cerebellum is a highly folded structure located at the posterior of the brain. It is important in maintaining posture and for coordinating head and eye movements, and is also involved in fine tuning of muscle movements and in learning motor skills1 Midbrain Pons Spinal cord Medulla oblongata PNS peripheral nervous system 1. Kandel et al. Principles of Neural Science. 4th Edition. 2000; 2. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009 5
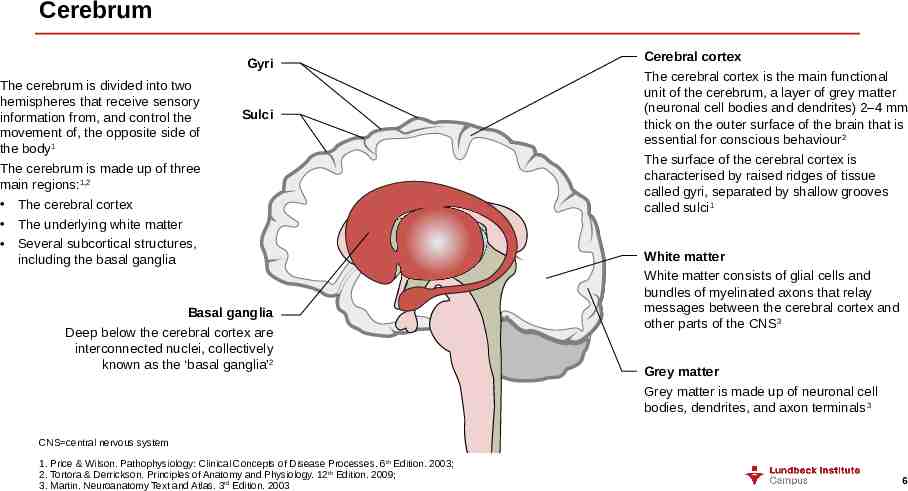
Cerebrum Gyri The cerebrum is divided into two hemispheres that receive sensory information from, and control the movement of, the opposite side of the body1 The cerebrum is made up of three main regions:1,2 The cerebral cortex The underlying white matter Several subcortical structures, including the basal ganglia Sulci Basal ganglia Deep below the cerebral cortex are interconnected nuclei, collectively known as the ‘basal ganglia’2 Cerebral cortex The cerebral cortex is the main functional unit of the cerebrum, a layer of grey matter (neuronal cell bodies and dendrites) 2–4 mm thick on the outer surface of the brain that is essential for conscious behaviour2 The surface of the cerebral cortex is characterised by raised ridges of tissue called gyri, separated by shallow grooves called sulci1 White matter White matter consists of glial cells and bundles of myelinated axons that relay messages between the cerebral cortex and other parts of the CNS3 Grey matter Grey matter is made up of neuronal cell bodies, dendrites, and axon terminals 3 CNS central nervous system 1. Price & Wilson. Pathophysiology: Clinical Concepts of Disease Processes. 6th Edition. 2003; 2. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009; 3. Martin. Neuroanatomy Text and Atlas. 3rd Edition. 2003 6
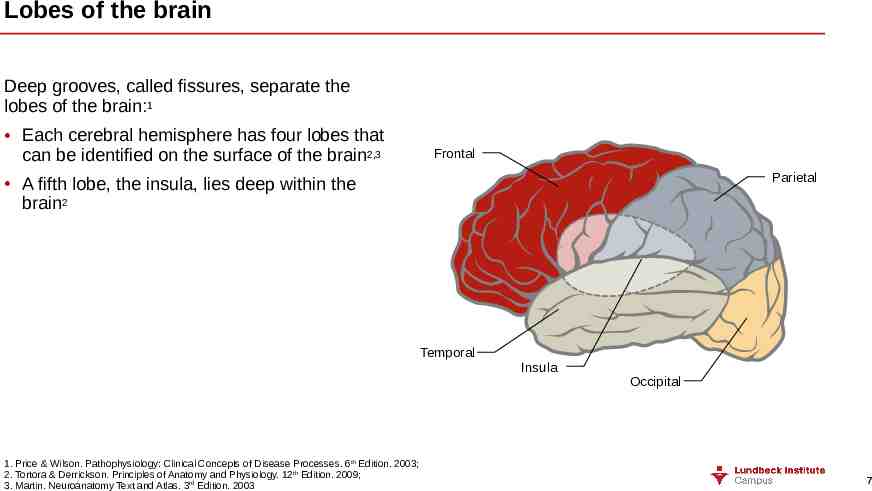
Lobes of the brain Deep grooves, called fissures, separate the lobes of the brain:1 Each cerebral hemisphere has four lobes that can be identified on the surface of the brain2,3 Frontal Parietal A fifth lobe, the insula, lies deep within the brain2 Temporal 1. Price & Wilson. Pathophysiology: Clinical Concepts of Disease Processes. 6th Edition. 2003; 2. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009; 3. Martin. Neuroanatomy Text and Atlas. 3rd Edition. 2003 Insula Occipital 7
Neurosynaptic transmission 8
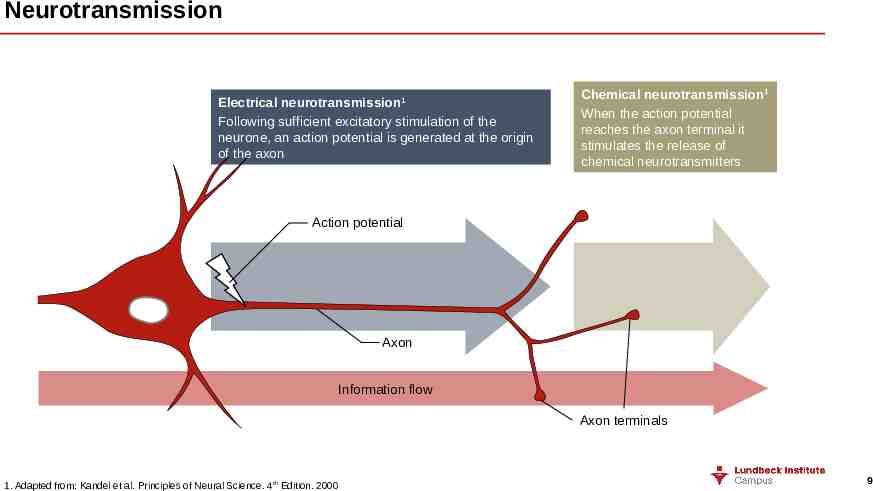
Neurotransmission Electrical neurotransmission 1 Following sufficient excitatory stimulation of the neurone, an action potential is generated at the origin of the axon Chemical neurotransmission1 When the action potential reaches the axon terminal it stimulates the release of chemical neurotransmitters Action potential Axon Information flow Axon terminals 1. Adapted from: Kandel et al. Principles of Neural Science. 4th Edition. 2000 9
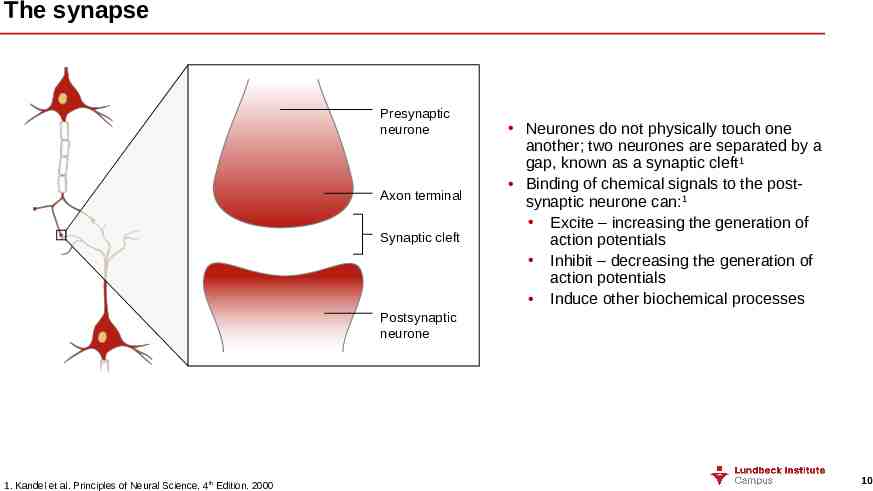
The synapse Presynaptic neurone Axon terminal Synaptic cleft Neurones do not physically touch one another; two neurones are separated by a gap, known as a synaptic cleft1 Binding of chemical signals to the postsynaptic neurone can:1 Excite – increasing the generation of action potentials Inhibit – decreasing the generation of action potentials Induce other biochemical processes Postsynaptic neurone 1. Kandel et al. Principles of Neural Science. 4th Edition. 2000 10
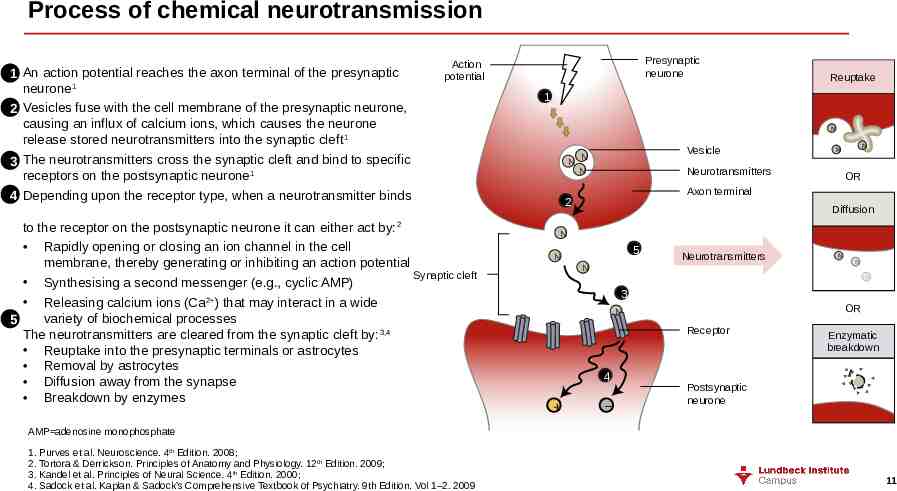
Process of chemical neurotransmission 1 2 3 4 An action potential reaches the axon terminal of the presynaptic neurone1 Vesicles fuse with the cell membrane of the presynaptic neurone, causing an influx of calcium ions, which causes the neurone release stored neurotransmitters into the synaptic cleft 1 The neurotransmitters cross the synaptic cleft and bind to specific receptors on the postsynaptic neurone 1 Depending upon the receptor type, when a neurotransmitter binds Presynaptic neurone Action potential to the receptor on the postsynaptic neurone it can either act by: Rapidly opening or closing an ion channel in the cell membrane, thereby generating or inhibiting an action potential Synaptic cleft Synthesising a second messenger (e.g., cyclic AMP) Releasing calcium ions (Ca2 ) that may interact in a wide variety of biochemical processes 5 The neurotransmitters are cleared from the synaptic cleft by: 3,4 Reuptake into the presynaptic terminals or astrocytes Removal by astrocytes Diffusion away from the synapse Breakdown by enzymes 2 Reuptake 1 N N Vesicle N Neurotransmitters N N N OR Axon terminal 2 Diffusion N 5 N Neurotransmitters N N N N 3 OR N Receptor 4 – Enzymatic breakdown Postsynaptic neurone AMP adenosine monophosphate 1. Purves et al. Neuroscience. 4th Edition. 2008; 2. Tortora & Derrickson. Principles of Anatomy and Physiology. 12th Edition. 2009; 3. Kandel et al. Principles of Neural Science. 4th Edition. 2000; 4. Sadock et al. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th Edition. Vol 1–2. 2009 11

Neurotransmitters 12
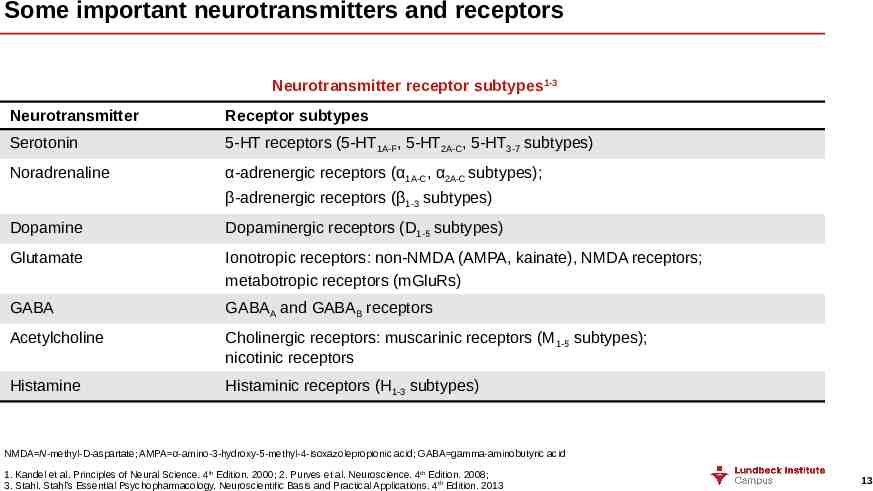
Some important neurotransmitters and receptors Neurotransmitter receptor subtypes1-3 Neurotransmitter Receptor subtypes Serotonin 5-HT receptors (5-HT1A-F, 5-HT2A-C, 5-HT3-7 subtypes) Noradrenaline α-adrenergic receptors (α1A-C, α2A-C subtypes); β-adrenergic receptors (β1-3 subtypes) Dopamine Dopaminergic receptors (D1-5 subtypes) Glutamate Ionotropic receptors: non-NMDA (AMPA, kainate), NMDA receptors; metabotropic receptors (mGluRs) GABA GABAA and GABAB receptors Acetylcholine Cholinergic receptors: muscarinic receptors (M 1-5 subtypes); nicotinic receptors Histamine Histaminic receptors (H1-3 subtypes) NMDA N-methyl-D-aspartate; AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA gamma-aminobutyric acid 1. Kandel et al. Principles of Neural Science. 4th Edition. 2000; 2. Purves et al. Neuroscience. 4th Edition. 2008; 3. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 13
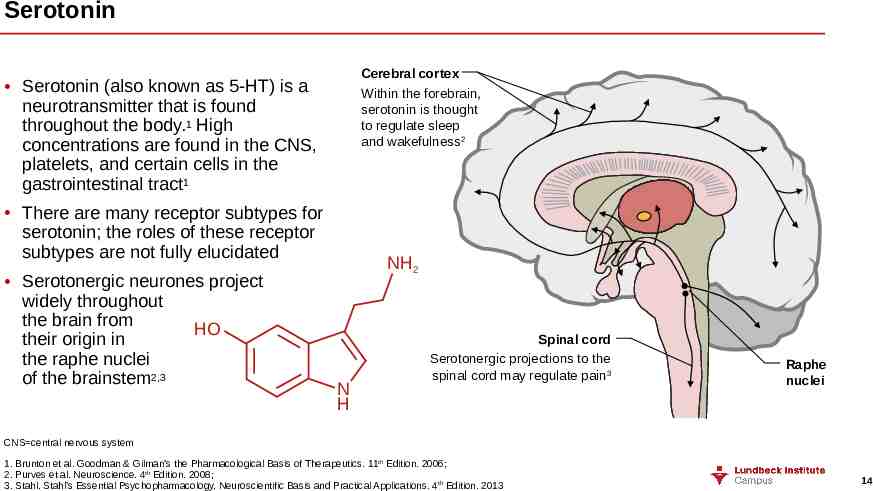
Serotonin Cerebral cortex Within the forebrain, serotonin is thought to regulate sleep and wakefulness2 Serotonin (also known as 5-HT) is a neurotransmitter that is found throughout the body.1 High concentrations are found in the CNS, platelets, and certain cells in the gastrointestinal tract1 There are many receptor subtypes for serotonin; the roles of these receptor subtypes are not fully elucidated Serotonergic neurones project widely throughout the brain from HO their origin in the raphe nuclei of the brainstem2,3 NH2 N H Spinal cord Serotonergic projections to the spinal cord may regulate pain3 Raphe nuclei CNS central nervous system 1. Brunton et al. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 11th Edition. 2006; 2. Purves et al. Neuroscience. 4th Edition. 2008; 3. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 14
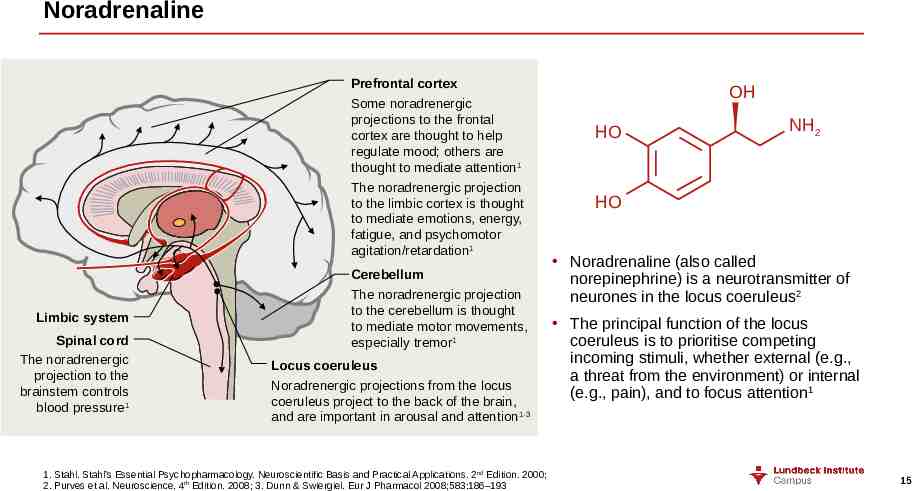
Noradrenaline Prefrontal cortex Some noradrenergic projections to the frontal cortex are thought to help regulate mood; others are thought to mediate attention1 The noradrenergic projection to the limbic cortex is thought to mediate emotions, energy, fatigue, and psychomotor agitation/retardation1 Limbic system Spinal cord The noradrenergic projection to the brainstem controls blood pressure1 Cerebellum The noradrenergic projection to the cerebellum is thought to mediate motor movements, especially tremor1 Locus coeruleus Noradrenergic projections from the locus coeruleus project to the back of the brain, and are important in arousal and attention 1-3 1. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 2nd Edition. 2000; 2. Purves et al. Neuroscience. 4th Edition. 2008; 3. Dunn & Swiergiel. Eur J Pharmacol 2008;583:186–193 OH HO NH2 HO Noradrenaline (also called norepinephrine) is a neurotransmitter of neurones in the locus coeruleus2 The principal function of the locus coeruleus is to prioritise competing incoming stimuli, whether external (e.g., a threat from the environment) or internal (e.g., pain), and to focus attention1 15
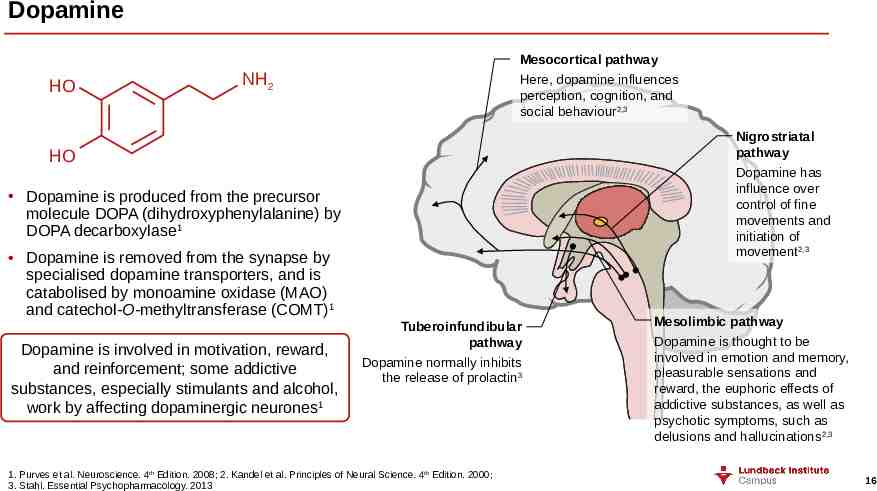
Dopamine HO Mesocortical pathway Here, dopamine influences perception, cognition, and social behaviour2,3 NH2 Nigrostriatal pathway Dopamine has influence over control of fine movements and initiation of movement2,3 HO Dopamine is produced from the precursor molecule DOPA (dihydroxyphenylalanine) by DOPA decarboxylase1 Dopamine is removed from the synapse by specialised dopamine transporters, and is catabolised by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT)1 Dopamine is involved in motivation, reward, and reinforcement; some addictive substances, especially stimulants and alcohol, work by affecting dopaminergic neurones1 Tuberoinfundibular pathway Dopamine normally inhibits the release of prolactin3 1. Purves et al. Neuroscience. 4th Edition. 2008; 2. Kandel et al. Principles of Neural Science. 4th Edition. 2000; 3. Stahl. Essential Psychopharmacology. 2013 Mesolimbic pathway Dopamine is thought to be involved in emotion and memory, pleasurable sensations and reward, the euphoric effects of addictive substances, as well as psychotic symptoms, such as delusions and hallucinations2,3 16
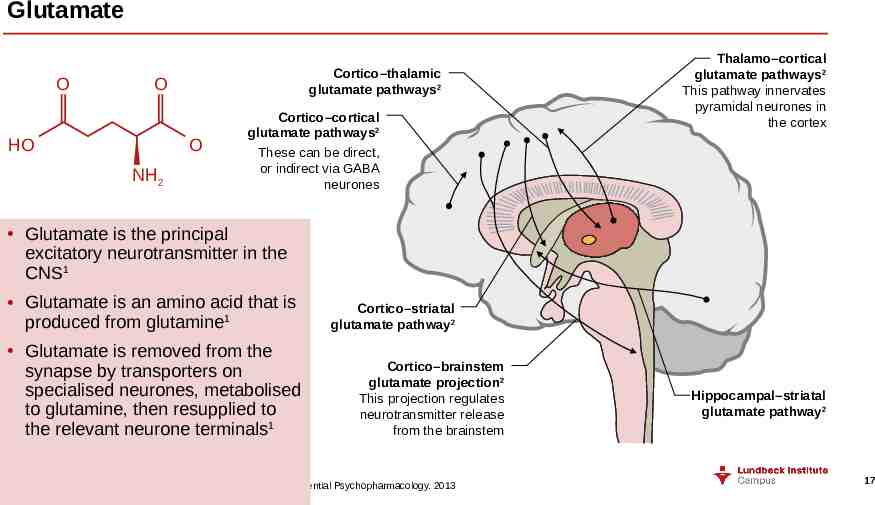
Glutamate O Cortico–thalamic glutamate pathways2 O HO O NH2 Cortico–cortical glutamate pathways2 These can be direct, or indirect via GABA neurones Thalamo–cortical glutamate pathways2 This pathway innervates pyramidal neurones in the cortex Glutamate is the principal excitatory neurotransmitter in the CNS1 Glutamate is an amino acid that is produced from glutamine1 Glutamate is removed from the synapse by transporters on specialised neurones, metabolised to glutamine, then resupplied to the relevant neurone terminals1 Cortico–striatal glutamate pathway2 Cortico–brainstem glutamate projection2 This projection regulates neurotransmitter release from the brainstem Hippocampal–striatal glutamate pathway2 CNS central nervous system 1. Purves et al. Neuroscience. 4th Edition. 2008; 2. Stahl. Stahl’s Essential Psychopharmacology. 2013 17
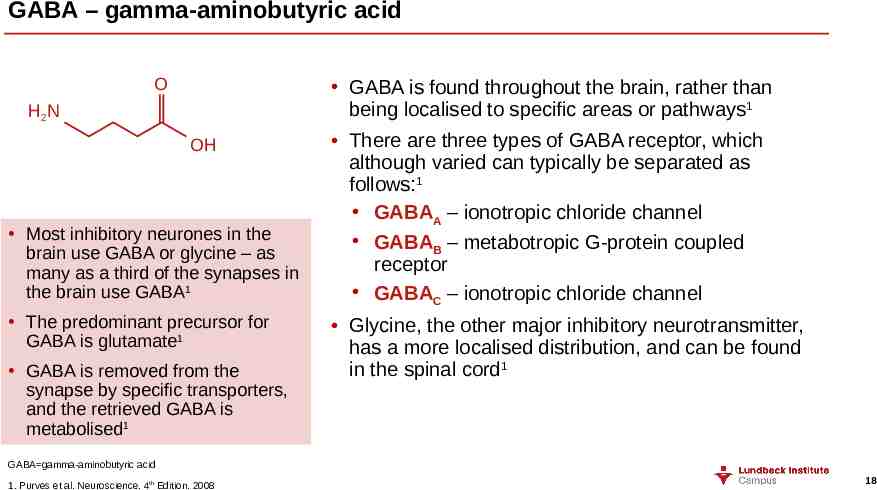
GABA – gamma-aminobutyric acid O GABA is found throughout the brain, rather than being localised to specific areas or pathways1 H2N OH Most inhibitory neurones in the brain use GABA or glycine – as many as a third of the synapses in the brain use GABA1 The predominant precursor for GABA is glutamate1 GABA is removed from the synapse by specific transporters, and the retrieved GABA is metabolised1 There are three types of GABA receptor, which although varied can typically be separated as follows:1 GABAA – ionotropic chloride channel GABAB – metabotropic G-protein coupled receptor GABAC – ionotropic chloride channel Glycine, the other major inhibitory neurotransmitter, has a more localised distribution, and can be found in the spinal cord1 GABA gamma-aminobutyric acid 1. Purves et al. Neuroscience. 4th Edition. 2008 18

Hypotheses about the underlying causes of depression 19
Neurochemical factors – the monoamine hypothesis The broad range of symptoms associated with major depressive disorder implicates several brain circuits and regions, and multiple neurotransmitter systems1 The three main monoamine systems associated with the pathophysiology of depression are serotonin, noradrenaline, and dopamine2 The ‘monoamine hypothesis’ postulates that a low activity of at least one of these neurotransmitters is responsible for the corresponding features of depression2 However, because of the inter-connectivity of the CNS, an attempt to enhance the levels of a single specific neurotransmitter may produce decreases in other neurotransmitters1 Treatment is generally aimed at counteracting this low or abnormal monoamine activity; increasing at least one of serotonin, noradrenaline, or dopamine1,2 CNS central nervous system 1. Blier. Int J Neuropsychopharmacol 2014;17(7):997–1008; 2. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 20
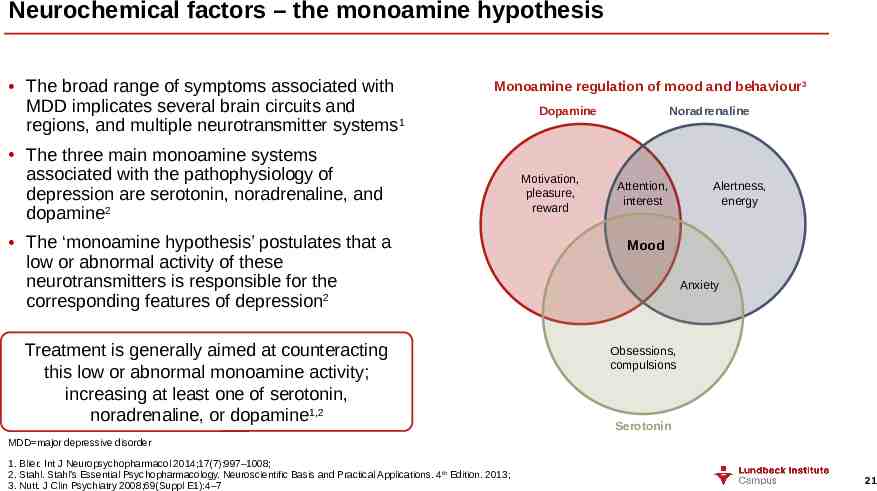
Neurochemical factors – the monoamine hypothesis The broad range of symptoms associated with MDD implicates several brain circuits and regions, and multiple neurotransmitter systems1 Monoamine regulation of mood and behaviour3 The three main monoamine systems associated with the pathophysiology of depression are serotonin, noradrenaline, and dopamine2 The ‘monoamine hypothesis’ postulates that a low or abnormal activity of these neurotransmitters is responsible for the corresponding features of depression2 Treatment is generally aimed at counteracting this low or abnormal monoamine activity; increasing at least one of serotonin, noradrenaline, or dopamine1,2 Dopamine Motivation, pleasure, reward Noradrenaline Attention, interest Alertness, energy Mood Anxiety Obsessions, compulsions Serotonin MDD major depressive disorder 1. Blier. Int J Neuropsychopharmacol 2014;17(7):997–1008; 2. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013; 3. Nutt. J Clin Psychiatry 2008;69(Suppl E1):4–7 21
Serotonin The serotonin system is an important target for the treatment of MDD1 In patients with MDD, low or abnormal serotonin activity is associated with anxiety, obsessions, compulsions, and low mood2 There is no single anomaly in the serotonin system that is common to the majority of patients with MDD; anomalies, when present, may only confer a slightly higher risk for developing MDD1 While an increase in serotonin levels can reduce some symptoms of MDD, it also has the effect of decreasing dopamine and noradrenaline activity1 Thus, an excess of serotonin can lead to fatigue, loss of interest or pleasure, insomnia, and sexual dysfunction3,4 MDD major depressive disorder 1. Blier & El Mansari. Philos Trans R Soc Lond B Biol Sci 2013;368(1615):20120536; 2. Nutt. J Clin Psychiatry 2008;69(Suppl E1):4–7; 3. Cassano & Fava. Ann Clin Psychiatry 2004;16(1):15–25; 4. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 22

Noradrenaline In patients with MDD, a low noradrenaline activity is associated with decreased alertness, low energy, inattention, loss of interest, concentration difficulties, and cognitive deficits1,2 Antidepressants that specifically increase noradrenergic activity are clinically effective, but do have a constellation of adverse effects related to the increase in noradrenaline2,3 Also, too much noradrenaline can lead to activation, anxiety, and sleep problems4 There is currently a lack of high-quality comparative studies comparing SSRIs with SNRIs; more evidence is needed to inform healthcare policy5 MDD major depressive disorder 1. Nutt. J Clin Psychiatry 2008;69(Suppl E1):4–7; 2. Moret & Briley. Neuropsychiatr Dis Treat 2011;7(Suppl 1):9–13; 3. Cassano & Fava. Ann Clin Psychiatry 2004;16(1):15–25; 4. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013; 5. Cipriani et al. Cochrane Database Syst Rev 2012;10:CD006533 23
Dopamine In patients with MDD, low or abnormal dopamine activity is implicated in some aspects of cognitive dysfunction, and in the symptoms of loss of motivation, loss of interest, and the inability to experience pleasure1,2 Antidepressants that enhance dopaminergic, as well as noradrenergic, activity appear to be beneficial in treating these symptoms (loss of motivation, loss of interest, the inability to experience pleasure, and fatigue)2 However, too much dopamine can lead to nausea, activation, and hyperkinetic movements such as tics and dyskinesias, and drugs with a dopaminergic component may have a greater liability for abuse3 MDD major depressive disorder 1. Nutt. J Clin Psychiatry 2008;69(Suppl E1):4–7; 2. Nutt et al. J Psychopharmacol 2007;21(5):461–471; 3. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 24

Glutamate As well as monoamine neurotransmitters, glutamate signalling has been linked to some of the pathophysiology of depression – glutamatergic abnormalities have been observed in the plasma, cerebrospinal fluid, and brain tissue of patients with mood disorders1,2 The brain can be considered a “glutamate machine”, regulated by GABA and other less commonly occurring neurotransmitters, including the monoamine neurotransmitters, therefore it is logical that “monoamine deficits” would lead to changes in glutamatergic signalling1 Glutamatergic agents are actively being studied in MDD, including modulators of different glutamate receptors. It is hoped that the future may see glutamate-modulating therapies as an option for patients with MDD3 1. Sanacora et al. Neuropharmacology 2012;62(1):63–77; 2. Sanacora et al. Nat Rev Drug Discov 2008;7(5):426–437; 3. Jaso et al. Curr Neuropharmacol 2017;15(1):57–70 25

GABA Dysregulation of GABA signalling has been linked to many mental disorders, including mood disorders1 Although it is difficult to study GABA levels in the brain of living patients, several studies have pointed to differences in certain key brain regions between patients with MDD and healthy controls2 The clinical picture is complicated by the association between GABA and stress:2 Stress is a risk factor for developing depression Stress leads to decreased levels of GABA within the brain 1. Chiapponi et al. Front Psychiatry 2016;7:61; 2. Luscher et al. Mol Psychiatry 2011;16(4):383–406 26
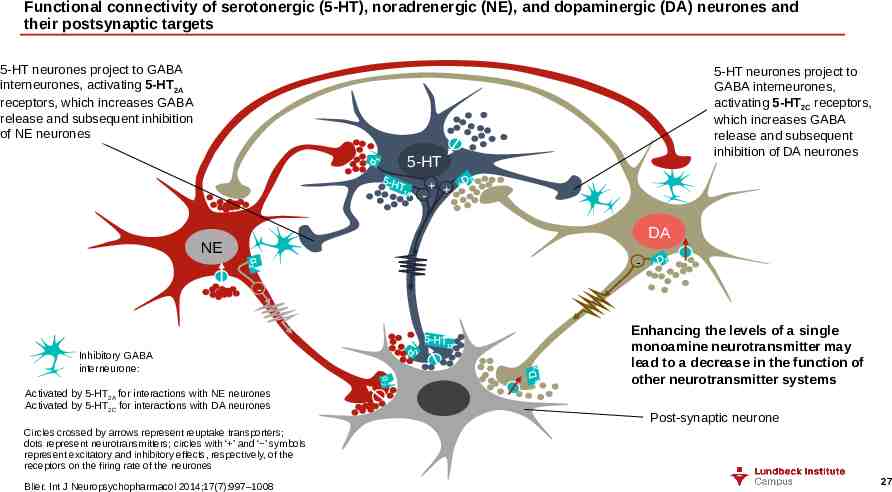
Functional connectivity of serotonergic (5-HT), noradrenergic (NE), and dopaminergic (DA) neurones and their postsynaptic targets 5-HT neurones project to GABA interneurones, activating 5-HT2A receptors, which increases GABA release and subsequent inhibition of NE neurones 5-HT neurones project to GABA interneurones, activating 5-HT2C receptors, which increases GABA release and subsequent inhibition of DA neurones 5-HT - DA NE - Inhibitory GABA interneurone: α2 Activated by 5-HT2A for interactions with NE neurones Activated by 5-HT2C for interactions with DA neurones Enhancing the levels of a single monoamine neurotransmitter may lead to a decrease in the function of other neurotransmitter systems Post-synaptic neurone Circles crossed by arrows represent reuptake transporters; dots represent neurotransmitters; circles with ‘ ’ and ‘ ’ symbols represent excitatory and inhibitory effects, respectively, of the receptors on the firing rate of the neurones Blier. Int J Neuropsychopharmacol 2014;17(7):997–1008 27
Functional brain abnormalities In many of the regions where structural abnormalities are apparent in patients with depression, the basal brain activity (as measured by cerebral blood flow and cerebral rate of glucose metabolism) is increased in the depressed phase relative to the remitted phase of MDD1 Note that the relationship between brain activity and depression symptom severity is complex and varies between brain structures:1 some brain areas, for example the dorsolateral prefrontal cortex, may show a decrease in activity2 The circuits implicated are those that normally regulate the evaluative, expressive, and experiential aspects of emotional behaviour1,3 CBT cognitive behavioural therapy; MDD major depressive disorder Linking changes in the brain to treatment Changes in the subgenual anterior cingulate cortex (part of the limbic system) or the ventromedial pre-frontal cortex (part of the frontal cortex) are produced from:1 Chronic treatment with an antidepressant Vagus nerve stimulation Deep brain stimulation Electroconvulsive therapy (ECT)4 and predict response to CBT5 Moreover, successful treatment of MDD leads to decreased activity in the amygdala (part of the limbic system)1 Data from structural and functional studies of patients with MDD highlight the importance of the limbic system and other circuitry1 1. Drevets et al. Brain Struct Funct 2008;213(1–2):93–118; 2. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013; 3. Phillips et al. Biol Psychiatry 2003;54(5):515–528; 4. Liu et al. Medicine (Baltimore) 2015;94(45):e2033; 5. Siegle et al. Am J Psychiatry 2006;163(4):735–738 28
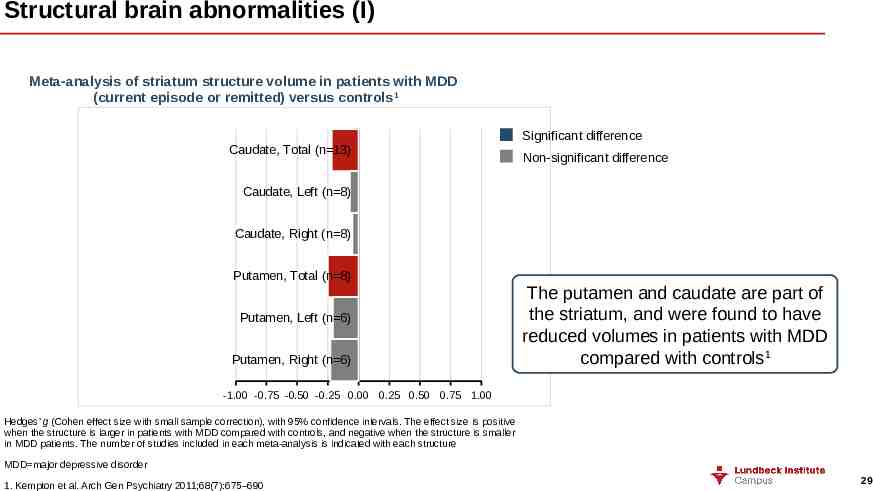
Structural brain abnormalities (I) Meta-analysis of striatum structure volume in patients with MDD (current episode or remitted) versus controls1 Caudate, Total (n 13) Significant difference Non-significant difference Caudate, Left (n 8) Caudate, Right (n 8) Putamen, Total (n 8) Putamen, Left (n 6) Putamen, Right (n 6) The putamen and caudate are part of the striatum, and were found to have reduced volumes in patients with MDD compared with controls1 -1.00 -0.75 -0.50 -0.25 0.00 0.25 0.50 0.75 1.00 Hedges’ g (Cohen effect size with small sample correction), with 95% confidence intervals. The effect size is positive when the structure is larger in patients with MDD compared with controls, and negative when the structure is smaller in MDD patients. The number of studies included in each meta-analysis is indicated with each structure MDD major depressive disorder 1. Kempton et al. Arch Gen Psychiatry 2011;68(7):675–690 29
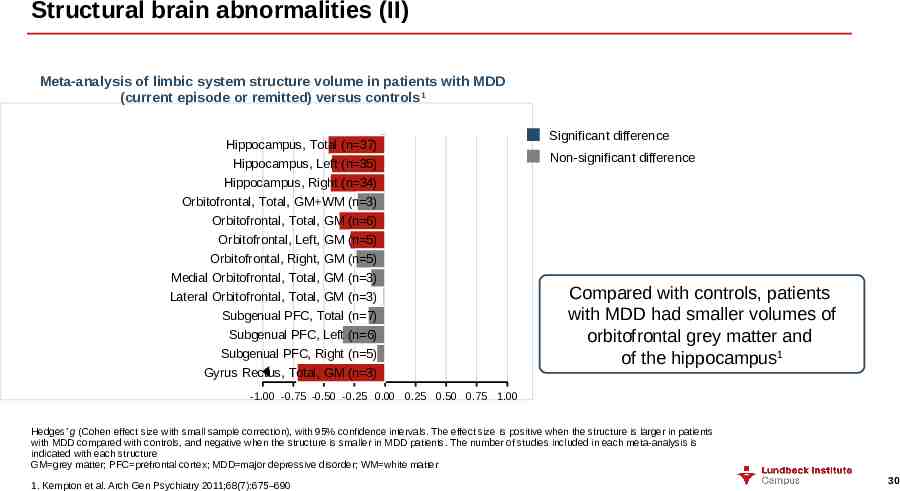
Structural brain abnormalities (II) Meta-analysis of limbic system structure volume in patients with MDD (current episode or remitted) versus controls1 Hippocampus, Total (n 37) Hippocampus, Left (n 35) Hippocampus, Right (n 34) Orbitofrontal, Total, GM WM (n 3) Orbitofrontal, Total, GM (n 6) Orbitofrontal, Left, GM (n 5) Orbitofrontal, Right, GM (n 5) Medial Orbitofrontal, Total, GM (n 3) Lateral Orbitofrontal, Total, GM (n 3) Subgenual PFC, Total (n 7) Subgenual PFC, Left (n 6) Subgenual PFC, Right (n 5) Gyrus Rectus, Total, GM (n 3) Significant difference Non-significant difference Compared with controls, patients with MDD had smaller volumes of orbitofrontal grey matter and of the hippocampus1 -1.00 -0.75 -0.50 -0.25 0.00 0.25 0.50 0.75 1.00 Hedges’ g (Cohen effect size with small sample correction), with 95% confidence intervals. The effect size is positive when the structure is larger in patients with MDD compared with controls, and negative when the structure is smaller in MDD patients. The number of studies included in each meta-analysis is indicated with each structure GM grey matter; PFC prefrontal cortex; MDD major depressive disorder; WM white matter 1. Kempton et al. Arch Gen Psychiatry 2011;68(7):675–690 30
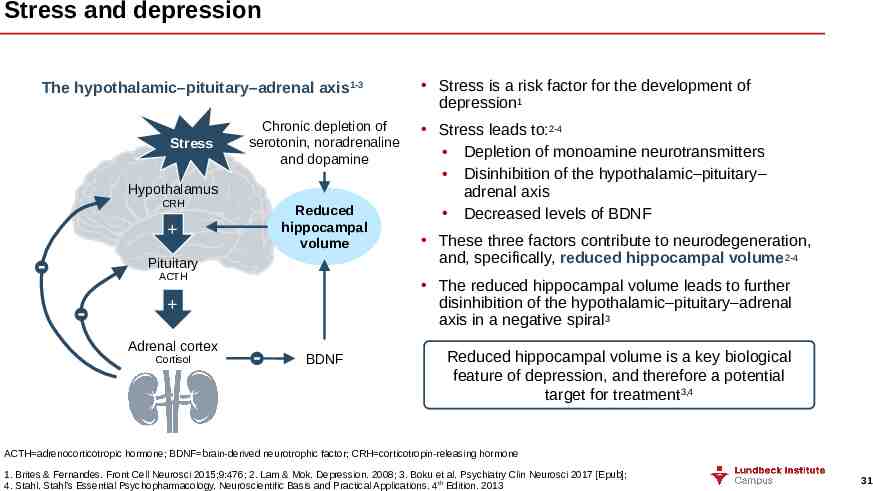
Stress and depression The hypothalamic–pituitary–adrenal axis1-3 Stress Chronic depletion of serotonin, noradrenaline and dopamine Hypothalamus CRH Reduced hippocampal volume Pituitary ACTH Cortisol Stress leads to:2-4 Depletion of monoamine neurotransmitters Disinhibition of the hypothalamic–pituitary– adrenal axis Decreased levels of BDNF These three factors contribute to neurodegeneration, and, specifically, reduced hippocampal volume2-4 The reduced hippocampal volume leads to further disinhibition of the hypothalamic–pituitary–adrenal axis in a negative spiral3 Adrenal cortex Stress is a risk factor for the development of depression1 BDNF Reduced hippocampal volume is a key biological feature of depression, and therefore a potential target for treatment3,4 ACTH adrenocorticotropic hormone; BDNF brain-derived neurotrophic factor; CRH corticotropin-releasing hormone 1. Brites & Fernandes. Front Cell Neurosci 2015;9:476; 2. Lam & Mok. Depression. 2008; 3. Boku et al. Psychiatry Clin Neurosci 2017 [Epub]; 4. Stahl. Stahl’s Essential Psychopharmacology. Neuroscientific Basis and Practical Applications. 4th Edition. 2013 31
Neuroinflammation Patients with MDD have characteristic alterations in the populations of microglia cells, which are the immune regulators of the brain1 Depression has been linked to increased immune signalling molecules – cytokines1,2 The hippocampus is a region of the brain with a notably high density of microglia; patients with MDD typically have smaller hippocampal volumes than healthy controls1,3 The problem is linking causality – does depression cause inflammation, or does inflammation cause depression?1 The link between neuroinflammation and depression could open up new routes for the treatment of depression1 MDD major depressive disorder 1. Brites & Fernandes. Front Cell Neurosci 2015;9:476; 2. Hurley & Tizabi. Neurotox Res 2013;23(2):131–144; 3. Kempton et al. Arch Gen Psychiatry 2011;68(7):675–690 32
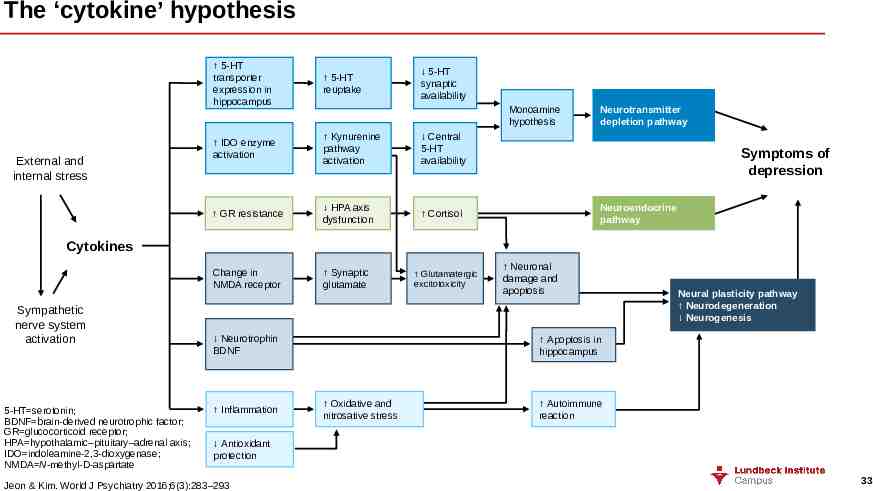
The ‘cytokine’ hypothesis External and internal stress 5-HT transporter expression in hippocampus 5-HT reuptake IDO enzyme activation Kynurenine pathway activation Central 5-HT availability GR resistance HPA axis dysfunction Cortisol Change in NMDA receptor Synaptic glutamate 5-HT synaptic availability Monoamine hypothesis Neurotransmitter depletion pathway Symptoms of depression Neuroendocrine pathway Cytokines Sympathetic nerve system activation 5-HT serotonin; BDNF brain-derived neurotrophic factor; GR glucocorticoid receptor; HPA hypothalamic–pituitary–adrenal axis; IDO indoleamine-2,3-dioxygenase; NMDA N-methyl-D-aspartate Neurotrophin BDNF Inflammation Glutamatergic excitotoxicity Neuronal damage and apoptosis Neural plasticity pathway Neurodegeneration Neurogenesis Apoptosis in hippocampus Oxidative and nitrosative stress Autoimmune reaction Antioxidant protection Jeon & Kim. World J Psychiatry 2016;6(3):283–293 33

Genetic factors 34
Studying the genetics of depression It has long been thought that MDD has a genetic component, based on data from family, twin, and adoption studies:1 Having a first-degree relative with MDD increases the risk of being diagnosed with MDD by 2.84 times2 In adoption studies, up to a three-fold increase in the rate of depression has been observed in the biological parents of a person with depression compared to the adoptive parents1 The heritability of MDD is approximately 40%3,4 Attempts to find a common genetic basis for MDD have had limited success4 MDD major depressive disorder 1. Sadock et al. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th Edition. Vol 1–2. 2009; 2. Sullivan et al. Am J Psychiatry 2000;157:1552–1562; 3. APA. DSM-5 2013; 4. Flint & Kendler. Neuron 2014;81(3):484–503 35
GWAS (genome-wide association studies) of MDD GWAS test differences in allele frequencies, between disease and control groups, at millions of common single nucleotide polymorphisms (SNPs) across the genome1 The PGC has undertaken large association studies into the genetics of ADHD, bipolar disorder, schizophrenia, autism, and MDD2 The PGC MDD working group analysed more than 1.2 million genetic variations in more than 18,000 volunteers2 Despite the power of the analysis, and the size of the sample, no genetic variation studied was found to be significant2 Several possibilities might confound attempts to unravel the genetics of MDD:3 There could be methodological problems with the construction of GWAS studies The genetics of MDD could potentially consist of multiple small-effect loci – these would be beneath the threshold of a GWAS MDD could potentially have a number of causes, and only some of these may interact with the underlying genetics or, MDD could simply be a condition with less of a genetic component than other diseases ADHD attention deficit hyperactivity disorder; GWAS genome-wide association study; MDD major depressive disorder; PGC Psychiatric Genomics Consortium 1. Mullins & Lewis. Curr Psychiatry Rep 2017;19(8):43; 2. MDD Working Group of the Psychiatric GWAS Consortium. Mol Psychiatry 2013;18(4):497–511; 3. Flint & Kendler. Neuron 2014;81(5):484–503 36
Big data and the potential of personal genetics Because the heritability of MDD is only approximately 40%, larger studies are required than have been attempted before, with more participants, in order to unlock the genetics of MDD1 In an attempt to reach these numbers, one research group combined the data set from the Psychiatric GWAS Consortium ( 18,000 cases and controls) with self-report data from the genetic testing company ‘23andme’ (which supplied genetic data from 300,000 cases and controls)1,2 Published in 2016, the results of this new analysis point to 17 variations in 15 regions of DNA as being associated with an increased risk of MDD2 This ground-breaking study shows the value of ‘big data’, and its great potential when properly harnessed – paving the way for the exploration of new avenues of the genetics of MDD1 DNA deoxyribonucleic acid; GWAS genome-wide association study; MDD major depressive disorder 1. Abbasi. JAMA 2017;317(1):14–16; 2. Hyde et al. Nat Genet 2016;48(9):1031–1036 37

Environmental factors 38
Environmental factors and MDD Environmental Adverse childhood experiences, especially multiple experiences of diverse types, constitute a set of potent risk factors for MDD 1-3 Stressful life events are well recognised as precipitating major depressive episodes2,4 Rather than one particular event, it is the accumulation of stressful negative life events that is the strongest predisposing factor to the development of depression 1 Stressors/course modifiers The risk of an individual developing depression is increased by virtually all other neurological disorders and general medical conditions 2 Substance use, anxiety disorder, and borderline personality disorder are among the most common of these2 The postpartum (shortly after childbirth) period is also associated with the development of depression1 Note that patients with other neurological disorders may present with symptoms that obscure and delay the diagnosis of MDD 2 In addition, major depressive episodes that develop against the background of another disorder often follow a more refractory course 2 MDD major depressive disorder 1. Sadock et al. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th Edition. Vol 1–2. 2009; 2. APA. DSM-5. 2013; 3. Chapman et al. J Affect Disord 2004;82(2):217–225; 4. Kendler et al. Am J Psychiatry 1999;156(6):837–841 39
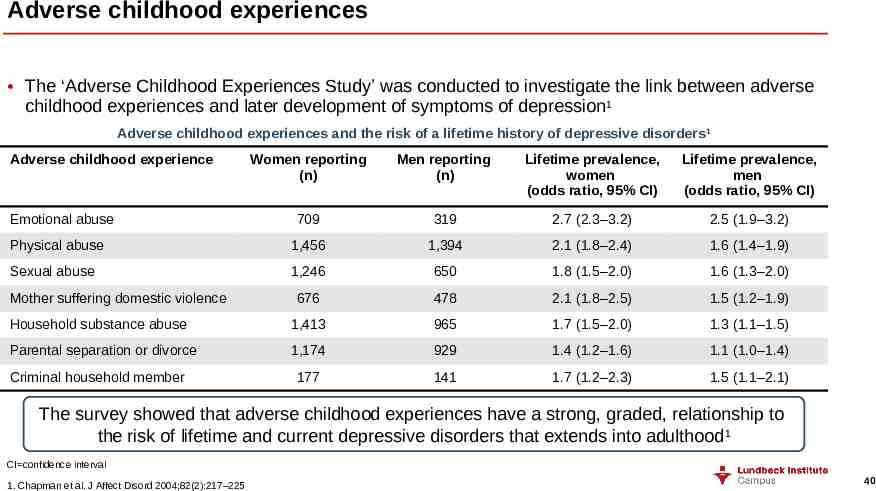
Adverse childhood experiences The ‘Adverse Childhood Experiences Study’ was conducted to investigate the link between adverse childhood experiences and later development of symptoms of depression1 Adverse childhood experiences and the risk of a lifetime history of depressive disorders 1 Adverse childhood experience Women reporting (n) Men reporting (n) Lifetime prevalence, women (odds ratio, 95% CI) Lifetime prevalence, men (odds ratio, 95% CI) 709 319 2.7 (2.3–3.2) 2.5 (1.9–3.2) Physical abuse 1,456 1,394 2.1 (1.8–2.4) 1.6 (1.4–1.9) Sexual abuse 1,246 650 1.8 (1.5–2.0) 1.6 (1.3–2.0) 676 478 2.1 (1.8–2.5) 1.5 (1.2–1.9) Household substance abuse 1,413 965 1.7 (1.5–2.0) 1.3 (1.1–1.5) Parental separation or divorce 1,174 929 1.4 (1.2–1.6) 1.1 (1.0–1.4) 177 141 1.7 (1.2–2.3) 1.5 (1.1–2.1) Emotional abuse Mother suffering domestic violence Criminal household member The survey showed that adverse childhood experiences have a strong, graded, relationship to the risk of lifetime and current depressive disorders that extends into adulthood1 CI confidence interval 1. Chapman et al. J Affect Disord 2004;82(2):217–225 40
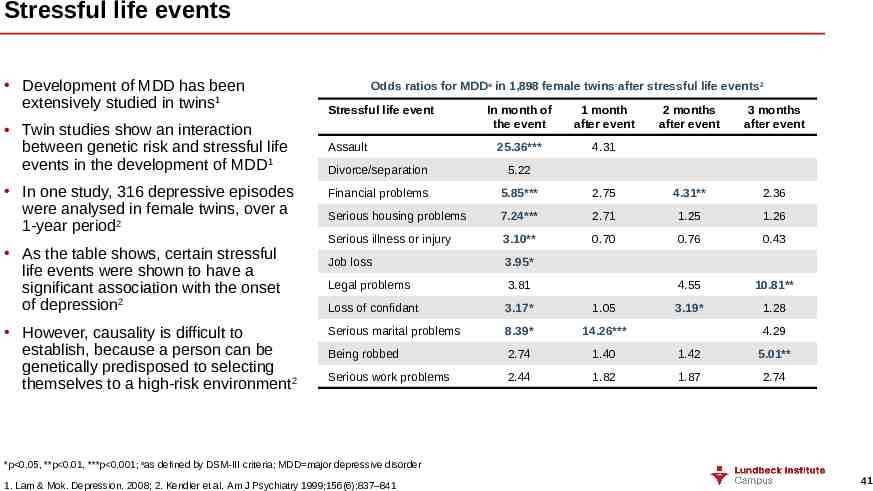
Stressful life events Development of MDD has been extensively studied in twins1 Twin studies show an interaction between genetic risk and stressful life events in the development of MDD1 In one study, 316 depressive episodes were analysed in female twins, over a 1-year period2 As the table shows, certain stressful life events were shown to have a significant association with the onset of depression2 However, causality is difficult to establish, because a person can be genetically predisposed to selecting themselves to a high-risk environment 2 Odds ratios for MDDa in 1,898 female twins after stressful life events 2 Stressful life event Assault In month of the event 1 month after event 25.36*** 4.31 2 months after event 3 months after event Divorce/separation 5.22 Financial problems 5.85*** 2.75 4.31** 2.36 Serious housing problems 7.24*** 2.71 1.25 1.26 Serious illness or injury 3.10** 0.70 0.76 0.43 Job loss 3.95* Legal problems 3.81 4.55 10.81** Loss of confidant 3.17* 1.05 3.19* 1.28 Serious marital problems 8.39* 14.26*** Being robbed 2.74 1.40 1.42 5.01** Serious work problems 2.44 1.82 1.87 2.74 4.29 *p 0.05, **p 0.01, ***p 0.001; aas defined by DSM-III criteria; MDD major depressive disorder 1. Lam & Mok. Depression. 2008; 2. Kendler et al. Am J Psychiatry 1999;156(6):837–841 41
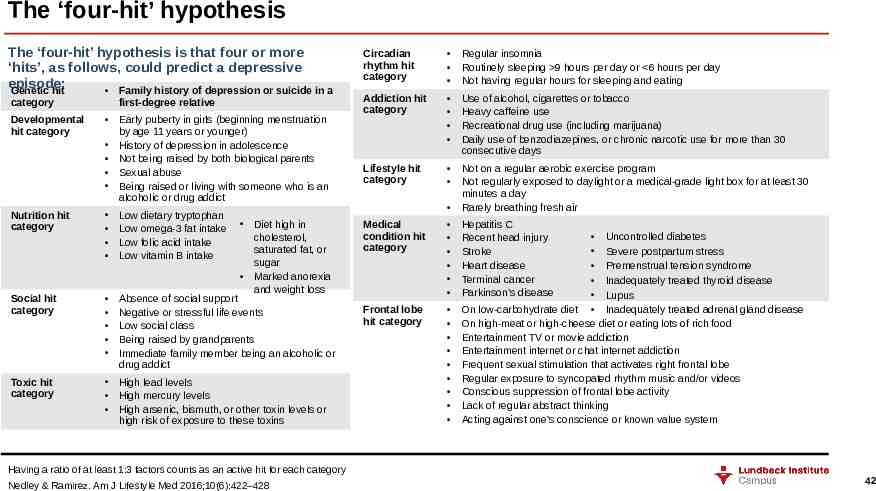
The ‘four-hit’ hypothesis The ‘four-hit’ hypothesis is that four or more ‘hits’, as follows, could predict a depressive episode: Genetic hit Family history of depression or suicide in a category Developmental hit category first-degree relative Early puberty in girls (beginning menstruation by age 11 years or younger) History of depression in adolescence Not being raised by both biological parents Sexual abuse Being raised or living with someone who is an alcoholic or drug addict Nutrition hit category Low dietary tryptophan Low omega-3 fat intake Low folic acid intake Low vitamin B intake Social hit category Absence of social support Negative or stressful life events Low social class Being raised by grandparents Immediate family member being an alcoholic or drug addict Toxic hit category High lead levels High mercury levels High arsenic, bismuth, or other toxin levels or high risk of exposure to these toxins Diet high in cholesterol, saturated fat, or sugar Marked anorexia and weight loss Circadian rhythm hit category Regular insomnia Routinely sleeping 9 hours per day or 6 hours per day Not having regular hours for sleeping and eating Addiction hit category Use of alcohol, cigarettes or tobacco Heavy caffeine use Recreational drug use (including marijuana) Daily use of benzodiazepines, or chronic narcotic use for more than 30 consecutive days Lifestyle hit category Not on a regular aerobic exercise program Not regularly exposed to daylight or a medical-grade light box for at least 30 minutes a day Rarely breathing fresh air Medical condition hit category Hepatitis C Recent head injury Stroke Heart disease Terminal cancer Parkinson’s disease Frontal lobe hit category Uncontrolled diabetes Severe postpartum stress Premenstrual tension syndrome Inadequately treated thyroid disease Lupus On low-carbohydrate diet Inadequately treated adrenal gland disease On high-meat or high-cheese diet or eating lots of rich food Entertainment TV or movie addiction Entertainment internet or chat internet addiction Frequent sexual stimulation that activates right frontal lobe Regular exposure to syncopated rhythm music and/or videos Conscious suppression of frontal lobe activity Lack of regular abstract thinking Acting against one’s conscience or known value system Having a ratio of at least 1:3 factors counts as an active hit for each category Nedley & Ramirez. Am J Lifestyle Med 2016;10(6):422–428 42






