Matter and Change IGCSE – HONORS 1 1
47 Slides8.76 MB

Matter and Change IGCSE - HONORS 1 1

All is Made of Particles Matter is everything that has mass and occupies space. All matter is made of particles. We know three types of state of the matter: –Solids –liquids –gases. –Plasma is a fourth state of the matter that we do not analyze at this level 2

The Particle Theory of Matter Matter is made up of tiny particles (Atoms, ions or Molecules) Particles of Matter are in constant motion. Particles of Matter are held together by very strong electric forces. Each substance has unique particles that are different from the particles of other substances Temperature affects the speed of the particles. The higher the temperature, the faster the speed of the particles. 7. Particles only stop moving when they reach the ABSOLUTE ZERO temperature (-273.15 C 0 K) 3

Solids Particles of solids are packed together and held in their postions by strong electrostatic forces. Particles of solids vibrate constantly due to their internal (Kinetic) energy but they cannot move from one place to another. The attraction forces are higher than the internal energy. (they cannot escape from the "NET" where they belong. The hotter the substance, the more Kinetic Energy it will have and the more movement in the particles. They have vibrational energy only. (They cannot rotate or move around) 4
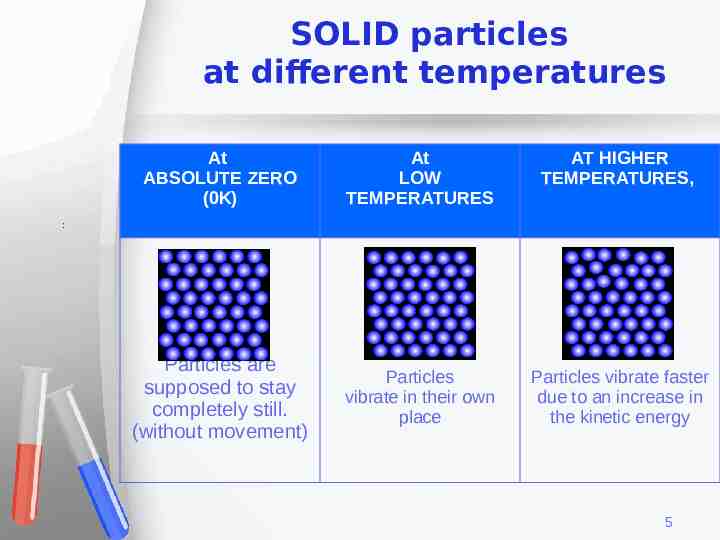
SOLID particles at different temperatures At ABSOLUTE ZERO (0K) At LOW TEMPERATURES AT HIGHER TEMPERATURES, Particles are supposed to stay completely still. (without movement) Particles vibrate in their own place Particles vibrate faster due to an increase in the kinetic energy : 5
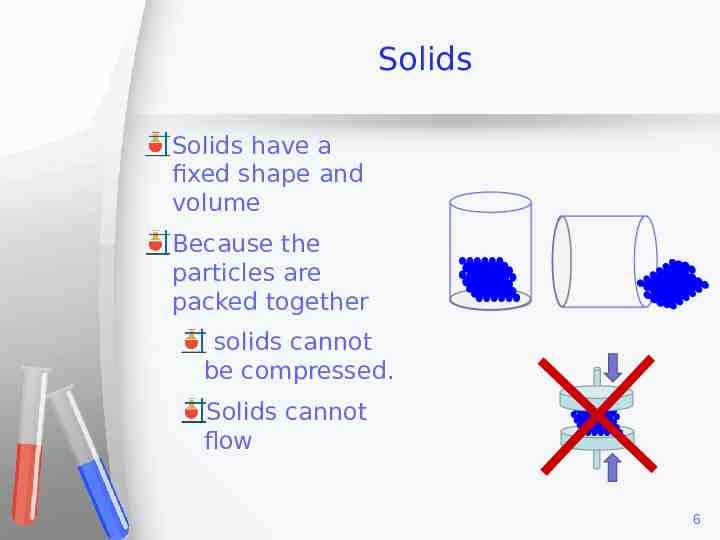
Solids Solids have a fixed shape and volume Because the particles are packed together solids cannot be compressed. Solids cannot flow 6
Molecules in Solids 7

Liquids Particles of liquids are kept together by forces of attraction weaker than those of solid particles. The internal energy of a liquid is bigger than the one in solids. They do not belong in a fixed position. They can move freely bot enough to escape the container. This movement is in all directions with different speeds, (RANDOM MOVEMENT). From time to time, some of the particles can reach enough energy to escape the container (evaporation) Free movement in the container allows liquids 8to

LIQUID particles Liquid particles move randomly but not with enough energy to escape the container. Particles bounce against each other and the container’s walls All particles move at different speeds, some of them, specially in the surface may have enough energy to escape the liquid. (evaporation) If the liquid is heated up to its boiling point, most of the particles will have enough energy to escape. This process is within the whole liquid, not only in the surface. (vaporization or9
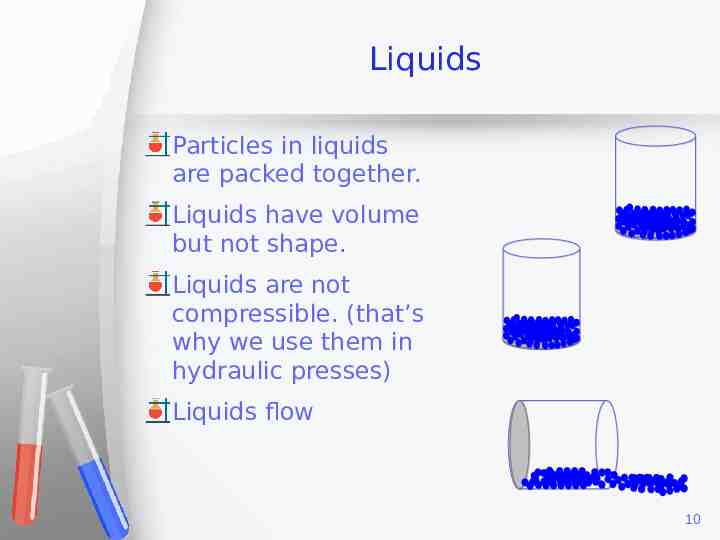
Liquids Particles in liquids are packed together. Liquids have volume but not shape. Liquids are not compressible. (that’s why we use them in hydraulic presses) Liquids flow 10

Molecules in Liquids 11

Gases The forces of attraction that hold gas particles together are very weak. The spaces between the particles are much larger than the spaces between solid and liquid particles. Gases movement are also random. (in any direction and with different speeds) Particles of gases can move freely within a container bumping against the walls and other particles. They move, rotate and vibrate at the same time. They can escape from a container very easily and they can put pressure on the side of the 12
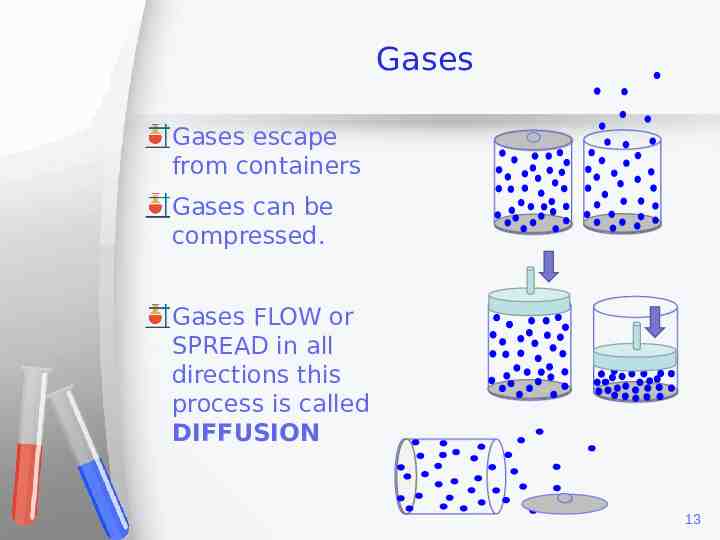
Gases Gases escape from containers Gases can be compressed. Gases FLOW or SPREAD in all directions this process is called DIFFUSION 13
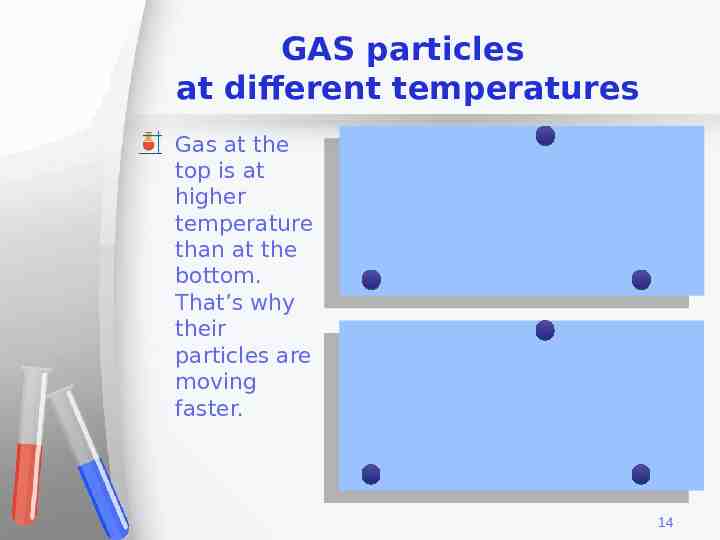
GAS particles at different temperatures Gas at the top is at higher temperature than at the bottom. That’s why their particles are moving faster. 14
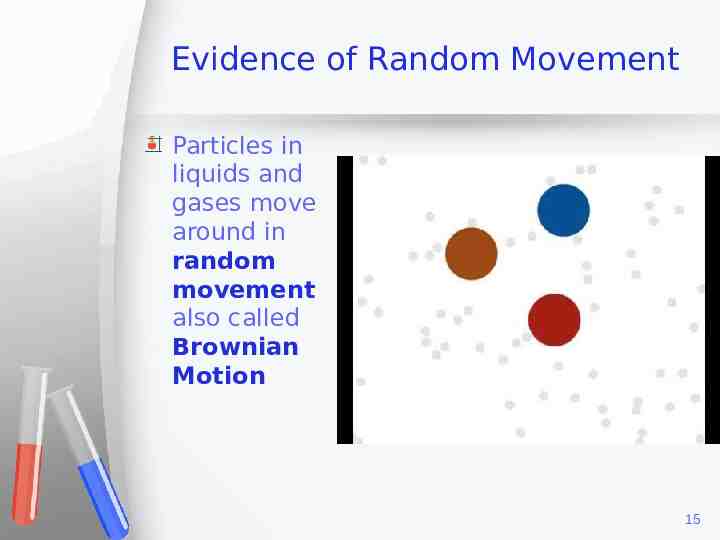
Evidence of Random Movement Particles in liquids and gases move around in random movement also called Brownian Motion 15

Why gases expand and contract? https://www.youtube.com/watch?v rMg1bmF5BVo 16
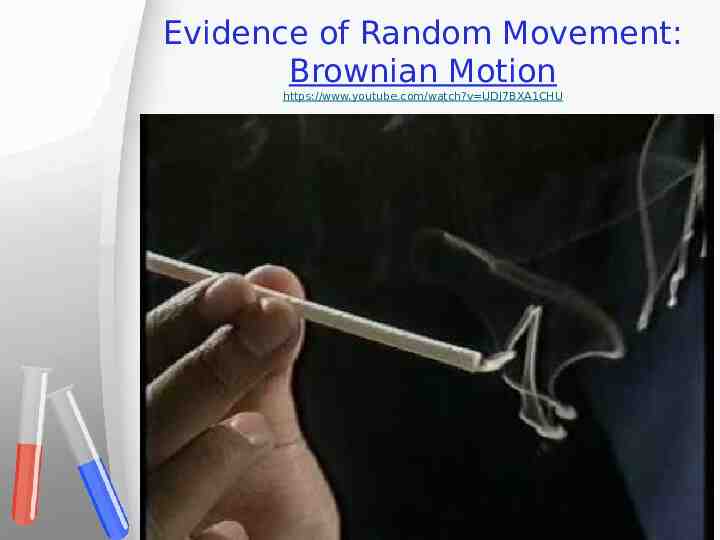
Evidence of Random Movement: Brownian Motion https://www.youtube.com/watch?v UDj7BXA1CHU 17

Consequences of Brownian Motion The movement of the particles inside a liquid or a gas makes the particles collide against each other and the container walls. If we place a crystal of a soluble solid inside the liquid, the particles will COLLIDE, DISSOLVE and SPREAD in that order. The Spreading of the substance is called DIFFUSION. 18

Diffusion and temperature Hot vs Cold water and Potassium Permanganate Crystals 19

Facts about diffusion Diffusion occurs in liquids and gases. Gases diffuse faster than liquids. Lighter gases diffuse faster than heavier ones. Solids do not diffuse unless they are volatile (they sublimate, like naphthalene or dry ice) Particles which diffuse can be: Molecules : CO2 gas or naphthalene (moth balls) Atoms: Any noble gas (Helium, Argon) Ions: an ionic compound soluble in water Potassium permanganate (K & MnO4-) Epson salts -magnesium sulfate (Mg2 & SO42-) Table salt - sodium chloride) (Na & Cl-) 20

Diffusion in Gases https://www.youtube.com/watch?v MwQvaXJGPA&feature youtu.be 21

Properties of Matter Properties of matter can be classified as EXTENSIVE or INTENSIVE properties. Extensive Property: depends on the amount of matter present. Example: weight. Intensive Property is which is INDEPENDENT of the amount of substance but depends on the identity of substance. Example: Boiling Temperature. Properties of matter can be classified as PHYSICAL or CHEMICAL properties. Physical Property: Can be observed without changing the identity of the substance. Example: mass, length, color, boiling point. Chemical Property: Can be observed only by testing the substance in a chemical reaction or change, and so, the substance will change its identity. Example: Flammability, reaction with 22

Classify the following Properties of Matter Property Intensive or Extensive Property Boiling Point Melting point Volume Flammability Mass Density Density Tarnish in air Conductivity Rusting Chemical or Physical 23

Changes of Matter Changes in matter can be classified as PHYSICAL or CHEMICAL changes. Physical change: It is the one that changes the form of a substance without changing the identity of the substance. Example: freezing, dissolving, boiling, cutting, etc. Chemical change: It is the one that changes the identity of the substance through a chemical reaction Example: rust, tarnish, burning, oxidation, etc. 24

Signs of a chemical change If a chemical change takes place you will observe at least one of the following signs. Change in color Production of smell Formation of Gas Formation of precipitate (solid) Change in light or heat 25

Classify the following Changes of Matter Change Physical of chemical Boiling alcohol Dissolving salt in water Burning a match Melting ice Grinding spices 26

States of Matter We already saw that there are three main states of matter. Solids Liquids Gases We also saw the properties of each state of the matter, like arrangement in particles, movement of particles at different temperatures, etc. Now we will talk about the changes that take place between those states. 27
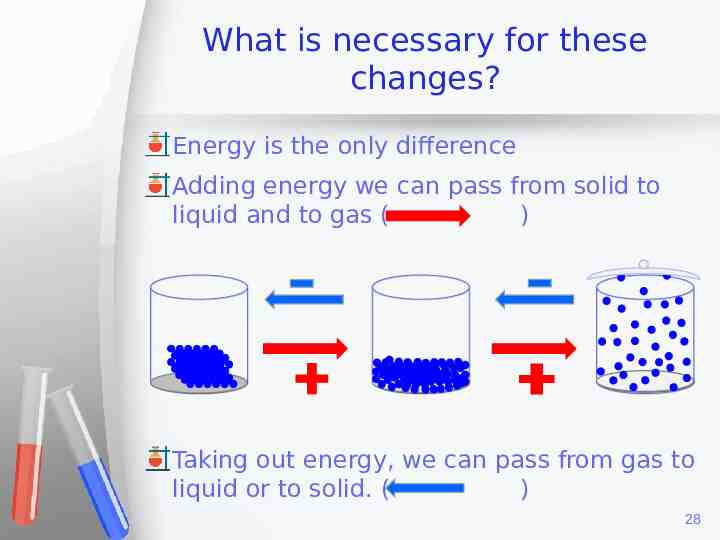
What is necessary for these changes? Energy is the only difference Adding energy we can pass from solid to liquid and to gas ( ) Taking out energy, we can pass from gas to liquid or to solid. ( ) 28
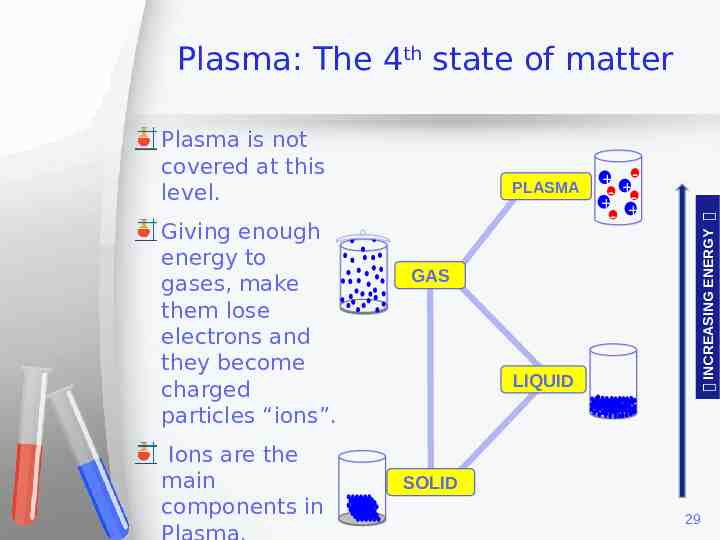
Plasma is not covered at this level. Giving enough energy to gases, make them lose electrons and they become charged particles “ions”. Ions are the main components in PLASMA GAS LIQUID - INCREASING ENERGY Plasma: The 4th state of matter SOLID 29
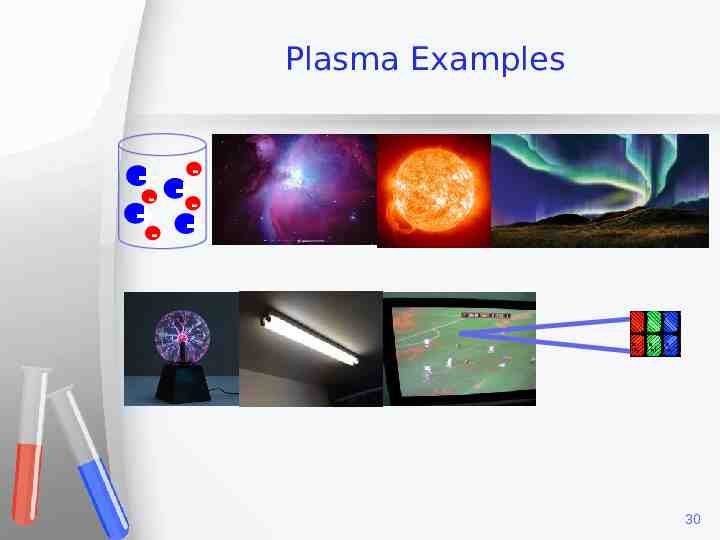
Plasma Examples - - 30
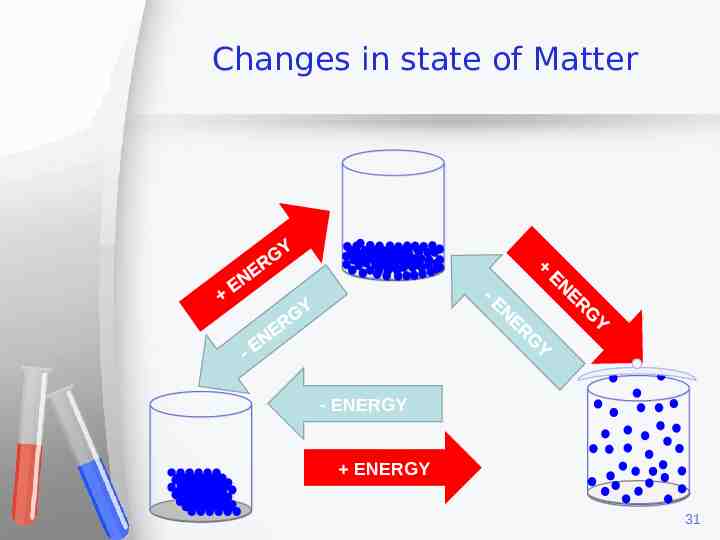
Changes in state of Matter - ENERGY ENERGY 31
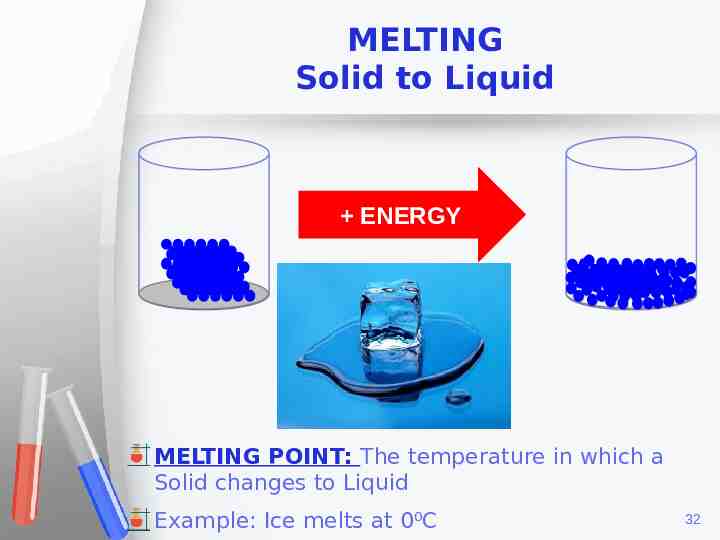
MELTING Solid to Liquid ENERGY MELTING POINT: The temperature in which a Solid changes to Liquid Example: Ice melts at 0⁰C 32
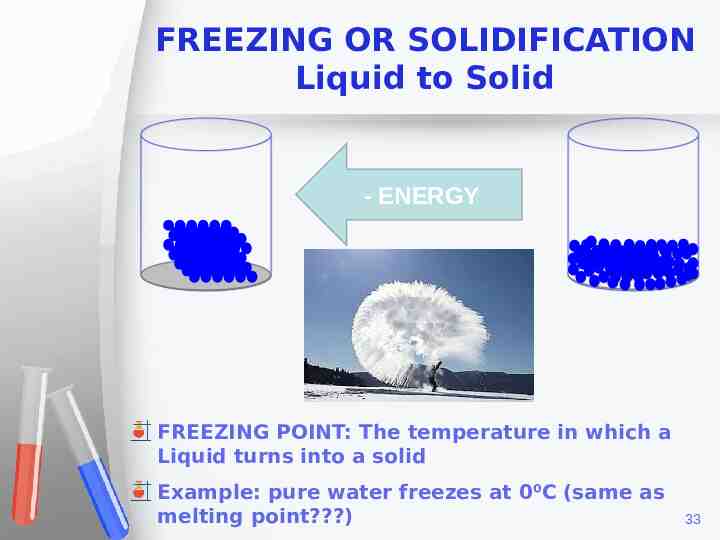
FREEZING OR SOLIDIFICATION Liquid to Solid - ENERGY FREEZING POINT: The temperature in which a Liquid turns into a solid Example: pure water freezes at 0⁰C (same as melting point?) 33
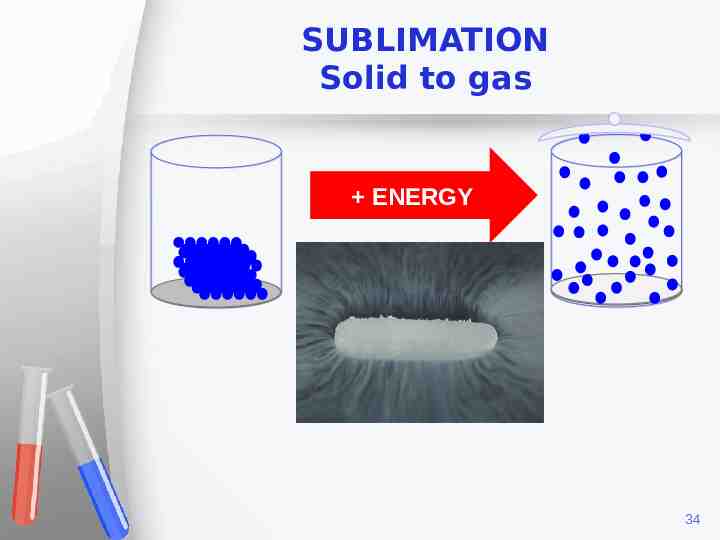
SUBLIMATION Solid to gas ENERGY 34

DEPOSITION Gas to Solid https://www.youtube.com/watch?v eqEzEghxYRM&feature youtu.be - ENERGY 35
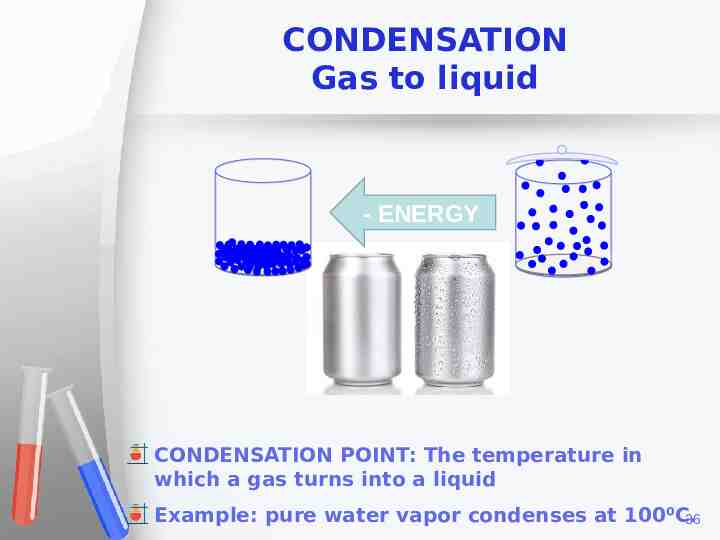
CONDENSATION Gas to liquid - ENERGY CONDENSATION POINT: The temperature in which a gas turns into a liquid Example: pure water vapor condenses at 100⁰C. 36
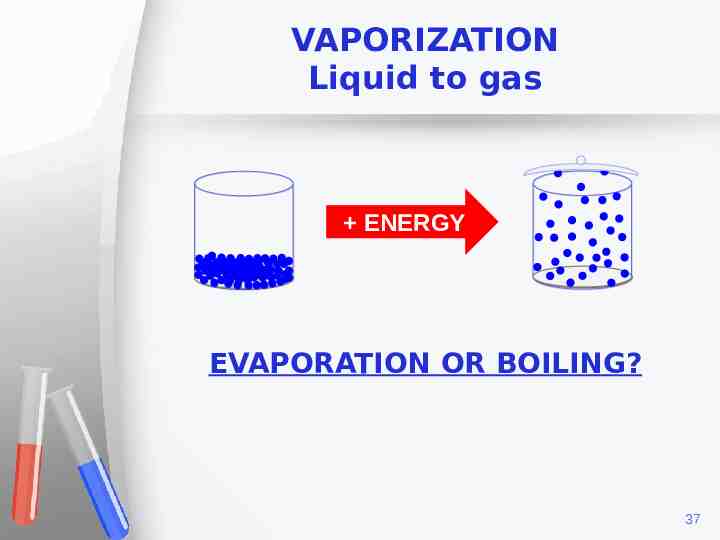
VAPORIZATION Liquid to gas ENERGY EVAPORATION OR BOILING? 37
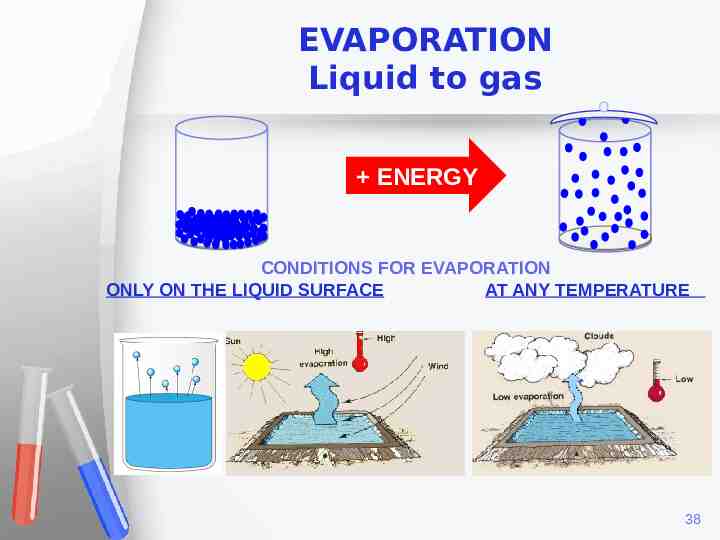
EVAPORATION Liquid to gas ENERGY CONDITIONS FOR EVAPORATION ONLY ON THE LIQUID SURFACE AT ANY TEMPERATURE 38
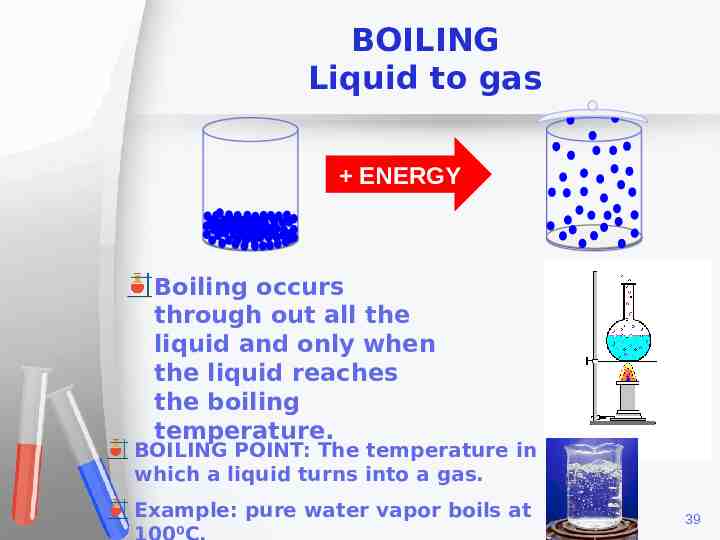
BOILING Liquid to gas ENERGY Boiling occurs through out all the liquid and only when the liquid reaches the boiling temperature. BOILING POINT: The temperature in which a liquid turns into a gas. Example: pure water vapor boils at 39
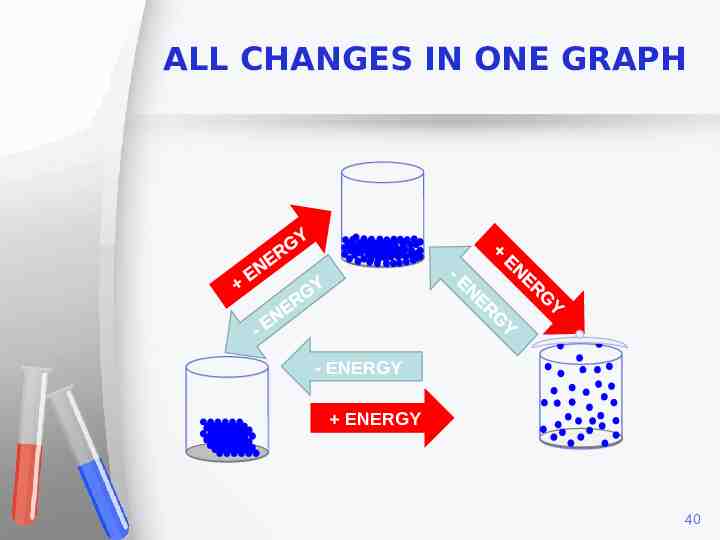
ALL CHANGES IN ONE GRAPH - ENERGY ENERGY 40
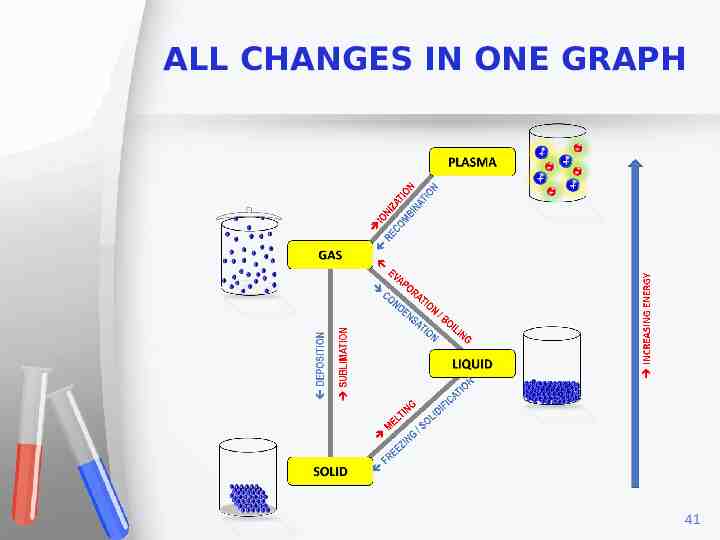
ALL CHANGES IN ONE GRAPH 41
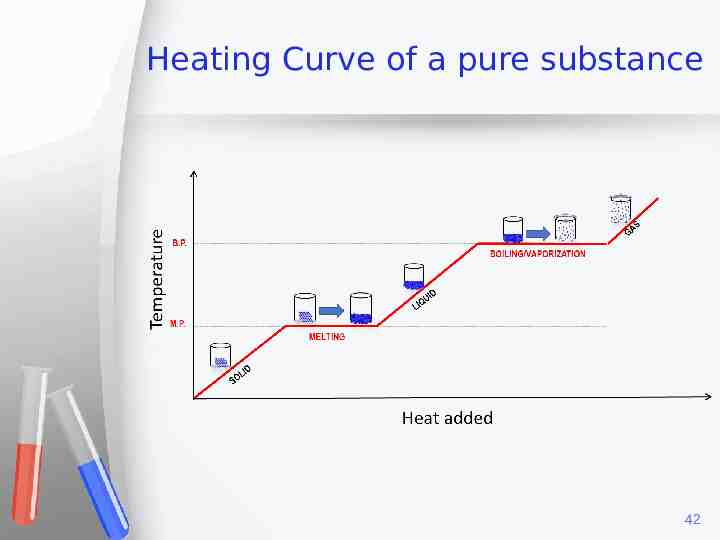
Heating Curve of a pure substance 42
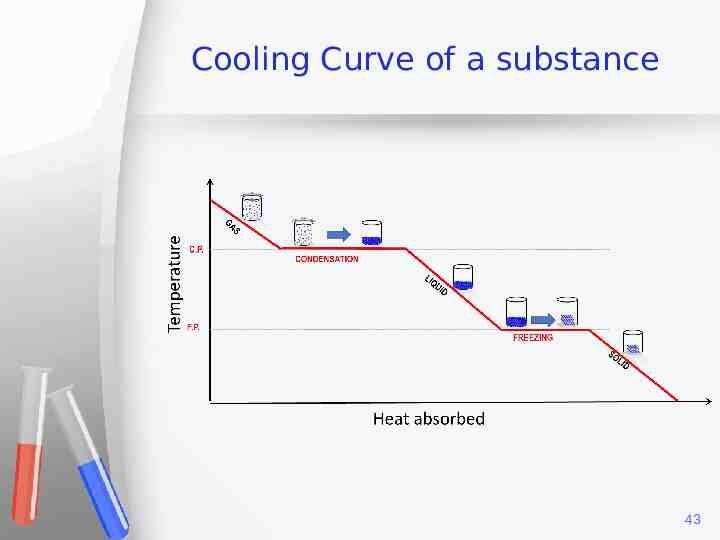
Cooling Curve of a substance 43
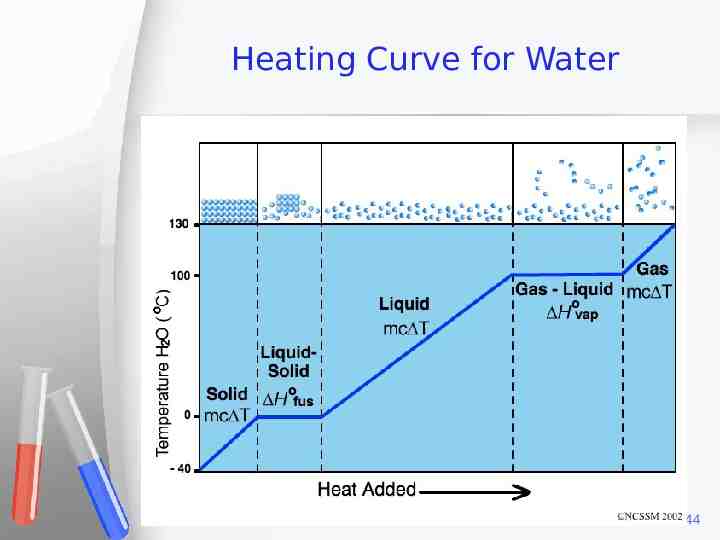
Heating Curve for Water 44
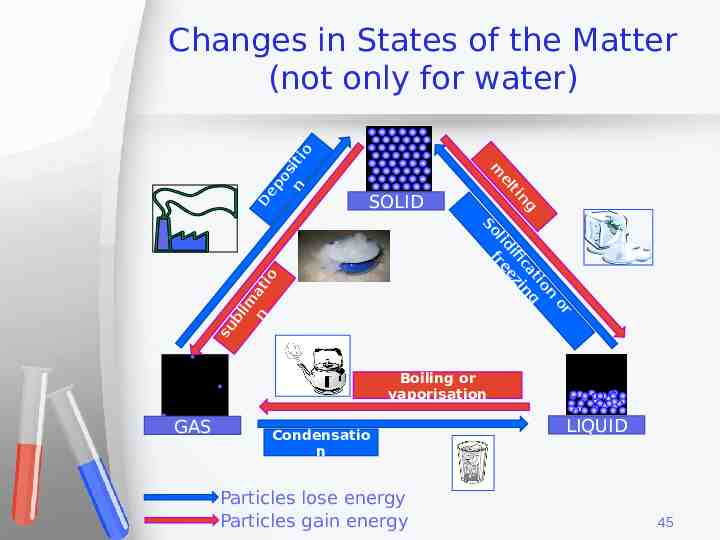
n su bl im at io SOLID or on ti g ca in ng ti ifi ez el lid fre So D m ep os n iti o Changes in States of the Matter (not only for water) Boiling or vaporisation GAS Condensatio n Particles lose energy Particles gain energy LIQUID 45
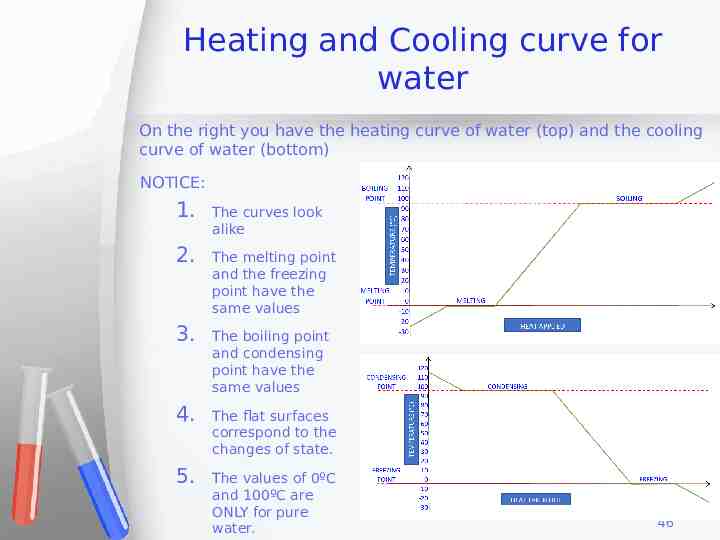
Heating and Cooling curve for water On the right you have the heating curve of water (top) and the cooling curve of water (bottom) NOTICE: 1. The curves look alike 2. The melting point and the freezing point have the same values 3. The boiling point and condensing point have the same values 4. The flat surfaces correspond to the changes of state. 5. The values of 0ºC and 100ºC are ONLY for pure water. 46
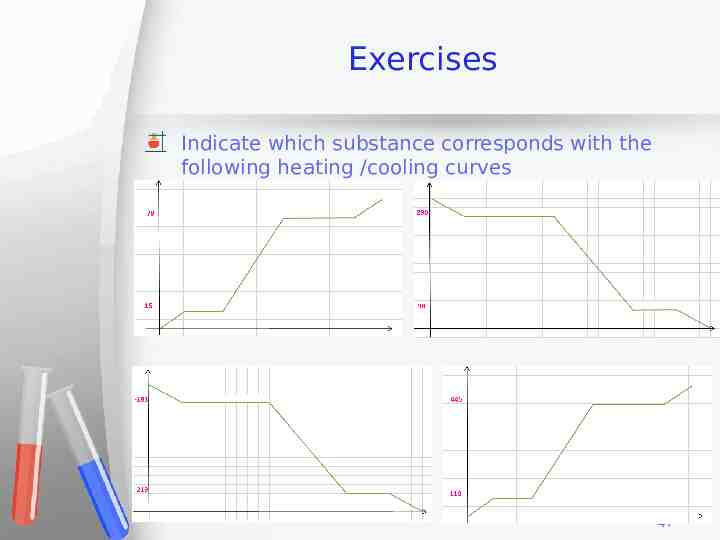
Exercises Indicate which substance corresponds with the following heating /cooling curves 47