Life Sciences Hub Wales Showcase Cell and Gene Therapy Special
21 Slides9.69 MB

Life Sciences Hub Wales Showcase Cell and Gene Therapy Special Interest Group

Martin Lamb Cath O’Brien Executive Vice President Director Welsh Blood Service Marketing & Sales TrakCel
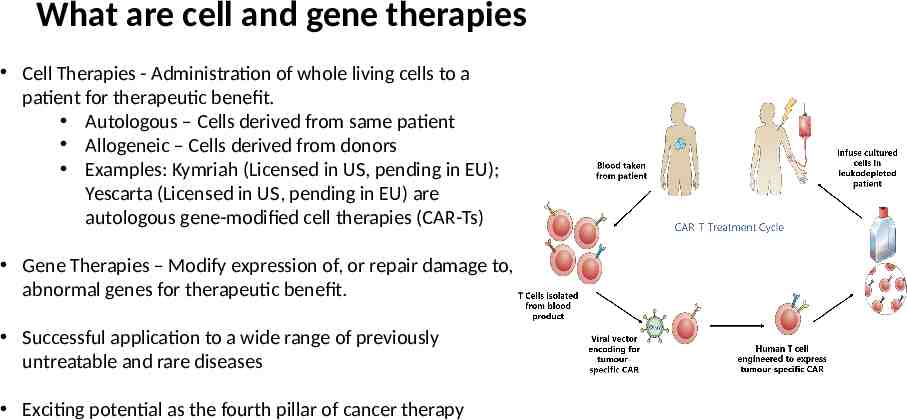
What are cell and gene therapies Cell Therapies - Administration of whole living cells to a patient for therapeutic benefit. Autologous – Cells derived from same patient Allogeneic – Cells derived from donors Examples: Kymriah (Licensed in US, pending in EU); Yescarta (Licensed in US, pending in EU) are autologous gene-modified cell therapies (CAR-Ts) Gene Therapies – Modify expression of, or repair damage to, abnormal genes for therapeutic benefit. Successful application to a wide range of previously untreatable and rare diseases Exciting potential as the fourth pillar of cancer therapy

Promise of durable and curative responses I Was Planning My Funeral. This Therapy Was My Last Chance. blogs.webmd.com/my-experience/2017/08/i-was-planning-myfuneral-this-therapy-was-my-last-chance.html dailymail.co.uk/health/article-3821187/Terminally-ill-patientcancer-free-raising-400-000-just-2-days-hope-treatment-US.html Terminally ill patient is now 'cancer free' after raising 400,000 for 'last-hope‘ Tx EmilyWhitehead.com evobiotalent.c om emilywhitehead.co m Landmark Phase I/II In Vivo Genome Editing for MPS II theatlantic.com/science/archive/2017/11/ sangamo-first-gene-editing-in-body/545960/ Gene editing clinical trial: Erasing sickle-cell disease
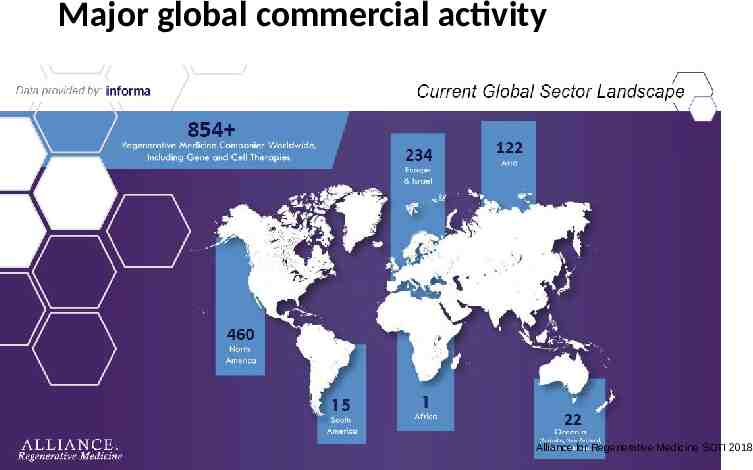
Major global commercial activity Alliance for Regenerative Medicine SOTI 2018
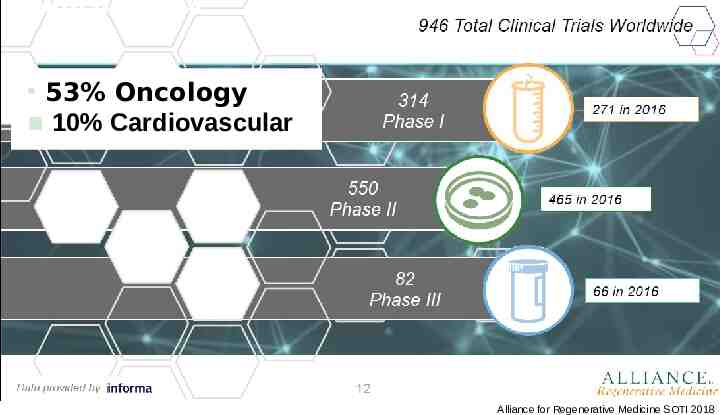
Increasing number of late stage clinical trials 53% Oncology 10% Cardiovascular Alliance for Regenerative Medicine SOTI 2018
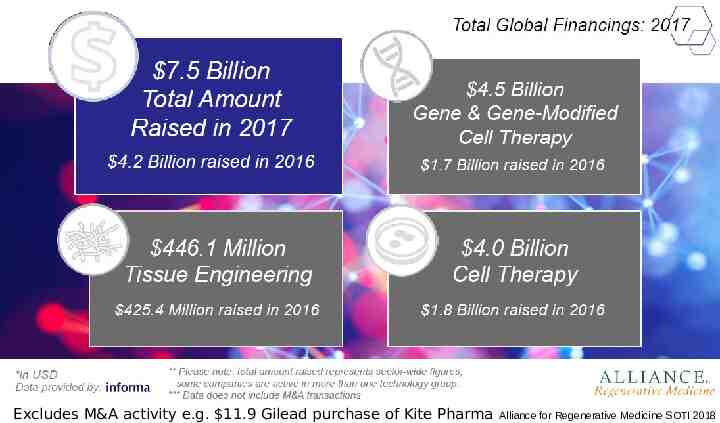
Increased investment Excludes M&A activity e.g. 11.9 Gilead purchase of Kite Pharma Alliance for Regenerative Medicine SOTI 2018
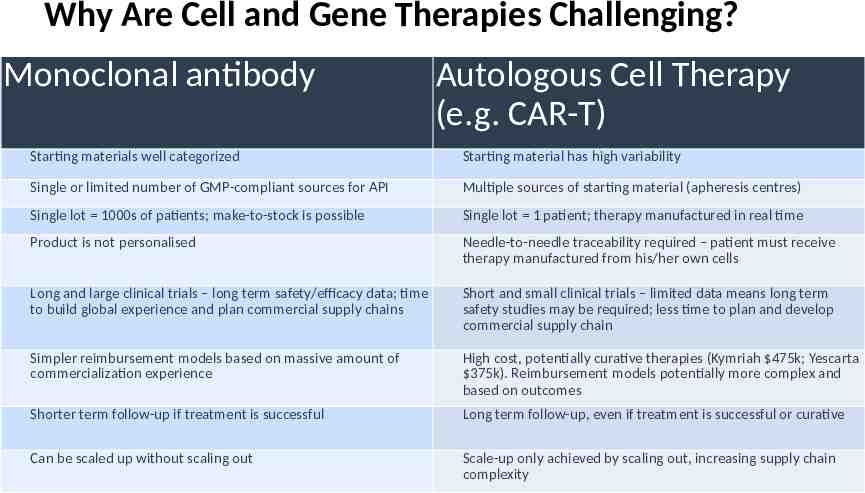
Why Are Cell and Gene Therapies Challenging? Monoclonal antibody Autologous Cell Therapy (e.g. CAR-T) Starting materials well categorized Starting material has high variability Single or limited number of GMP-compliant sources for API Multiple sources of starting material (apheresis centres) Single lot 1000s of patients; make-to-stock is possible Single lot 1 patient; therapy manufactured in real time Product is not personalised Needle-to-needle traceability required – patient must receive therapy manufactured from his/her own cells Long and large clinical trials – long term safety/efficacy data; time Short and small clinical trials – limited data means long term to build global experience and plan commercial supply chains safety studies may be required; less time to plan and develop commercial supply chain Simpler reimbursement models based on massive amount of commercialization experience High cost, potentially curative therapies (Kymriah 475k; Yescarta 375k). Reimbursement models potentially more complex and based on outcomes Shorter term follow-up if treatment is successful Long term follow-up, even if treatment is successful or curative Can be scaled up without scaling out Scale-up only achieved by scaling out, increasing supply chain complexity
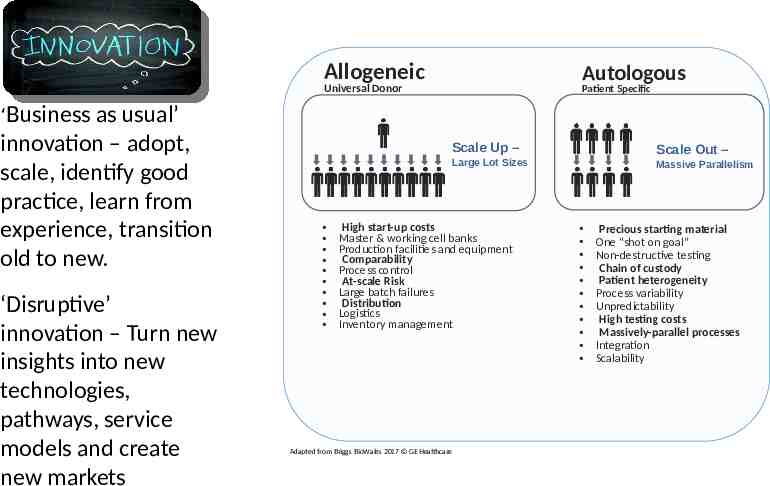
Production challenges require innovative solutions Allogeneic Autologous Universal Donor Patient Specific ‘Business as usual’ innovation – adopt, scale, identify good practice, learn from experience, transition old to new. ‘Disruptive’ innovation – Turn new insights into new technologies, pathways, service models and create new markets Scale Up – Scale Out – Large Lot Sizes High start-up costs Master & working cell banks Production facilities and equipment Comparability Process control At-scale Risk Large batch failures Distribution Logistics Inventory management Adapted from Briggs BioWales 2017 GE Healthcare Massive Parallelism Precious starting material One “shot on goal” Non-destructive testing Chain of custody Patient heterogeneity Process variability Unpredictability High testing costs Massively-parallel processes Integration Scalability

The Context – Health and Economy House of Lords, Science and Technology Committee Regenerative Medicine Report 2013 Medicines Manufacturing Industry Partnership Advanced Therapy Action Plan Building on our potential; a UK pathway for regenerative medicine A report from the Regenerative Medicine Expert Group Industrial Strategy – A report to the Government from the life sciences sector Innovate UK – Industrial Strategy Challenge Fund
Background Welsh government has expressed an intent to develop a strategy for Regenerative Medicine (Cell and Gene Therapy) Strategy administered by National Oversight Group (NOG) lead by Chief Medical Officer (CMO) Frank Atherton The Welsh Blood Service support the development of strategy for Regenerative medicine (Cell and Gene Therapy) The Special Interest Group (SIG) was established in 2016 as a stakeholder and advisory group 12

SIG: Broad and enthusiastic engagement

Life Sciences Hub Cell and Gene Therapy Special Interest Group Life Sciences Hub: Cell and Gene Therapy Special Interest Group, A crucible of ideas for the Welsh Cell and Gene Therapy Industry. A forum for collaboration between leaders in the Welsh Cell and Gene Therapy Industry, discussing all aspects of this industry – Academia, Health Service, Government, Private Sector

Life Sciences Hub Cell and Gene Therapy Special Interest Group More than a talking shop – developing solutions for specific barriers to Wales becoming a world leader in cell and gene therapies: Skills Resources Indigenous industry development Inward investment Outlining training and education needs to Universities and Colleges with an aim to produce a workforce in Wales suitable for the Cell and Gene Therapy industry, driving Wales’ knowledge-based economy forward. The Life Sciences Hub had built a network of private and public sector companies with established relationships and were able to assist with the formulation of consortium in answer to an Innovate UK competition.

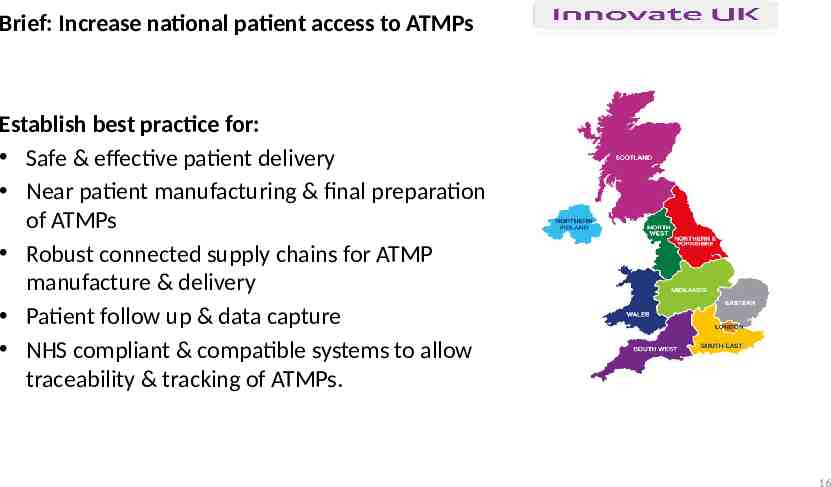
Advanced Therapies Treatment Centre Brief: Increase national patient access to ATMPs Establish best practice for: Safe & effective patient delivery Near patient manufacturing & final preparation of ATMPs Robust connected supply chains for ATMP manufacture & delivery Patient follow up & data capture NHS compliant & compatible systems to allow traceability & tracking of ATMPs. 16
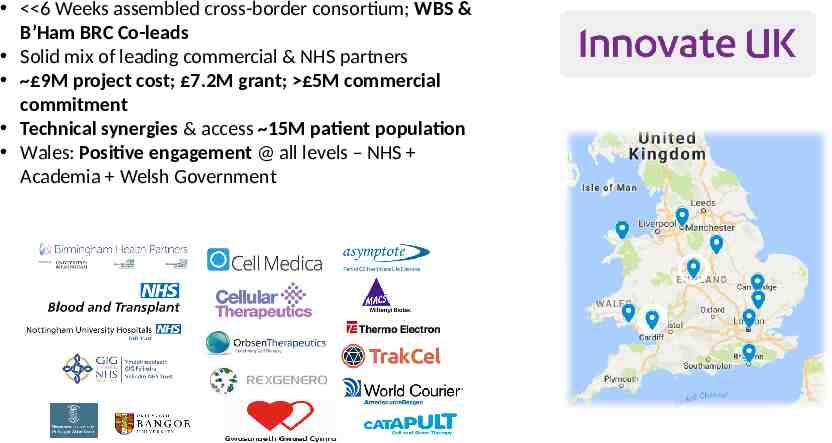
Midlands & Wales ATTC 6 Weeks assembled cross-border consortium; WBS & B’Ham BRC Co-leads Solid mix of leading commercial & NHS partners 9M project cost; 7.2M grant; 5M commercial commitment Technical synergies & access 15M patient population Wales: Positive engagement @ all levels – NHS Academia Welsh Government
Aims of Midlands Wales Advanced Therapy Treatment ATTC Selection Centre Set up a network of hospitals with medical staff trained to receive and administer ATMPs Build seamless supply chains that ensure that 'living medicines' remain healthy and effective as they are moved from the production laboratory to the bedside Put in place the IT systems to manage the end-to-end process Validate this new infrastructure using real ATMPs Deliver a programme that uses this infrastructure to speed up the testing of ATMPs in clinical trials Set-up protocols to test whether the cost of a new ATMP is justified by its clinical effectiveness As a result Wales and Welsh patients will have access to advanced therapies sooner!

Summary Transformative therapies require transformative thinking Incredible pace of change Partnerships and broad collaboration are essential ATTC bid – just a start Example of strength in partnership between NHS, academia and industry Next steps Deliver on ATTC Finalise strategic proposals Deliver for Wales SIG support and new ventures
Company Background TrakCel was established in 2012 by a combination of Pharma cold chain, information technology, and clinical development experts focused on the Cell and Gene Therapy market. TrakCel was the first provider of integrated orchestration software for the precise management, control and tracking of cell therapy products. TrakCel employs more than 60 members of staff, most of whom are based an the company’s headquarters in Cardiff, UK. TrakCel has an additional office located in USA.
TrakCel’s Software Cell Orchestration Platform (COP) software delivering: Regulatory compliance (COI) and patient safety Full needle-needle visibility and control (COC) Automated scheduling of activities between supply chain partners Workflow-driven consistent processing Real time communication and alerts Role-based functionality and information access – physicians, nurses, patients/families, supply chain partners Cloud- or client-hosted