Heats of Combustion, Heats of Formation, Heats of Hydrogenation
20 Slides719.00 KB

Heats of Combustion, Heats of Formation, Heats of Hydrogenation and Bond Dissociation Energies

Hess’s Law (1840) The total heat liberated in a series of chemical reactions is equal to the sum of the heats liberated in the individual steps. The heat liberated, Ho, (enthalpy) is a state function. Germain Henri Hess (1802 - 1850) State functions are independent of path.
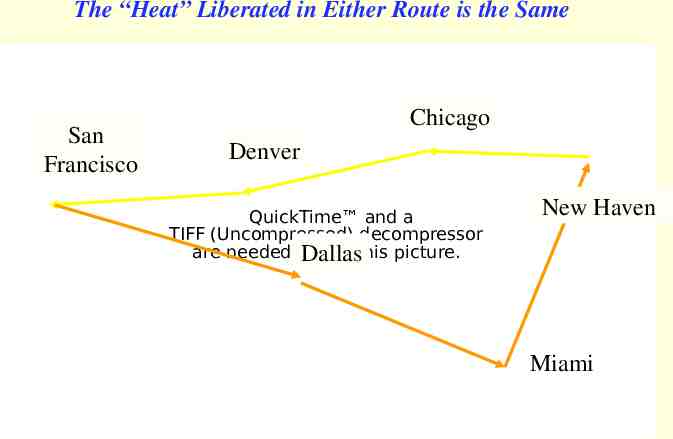
The “Heat” Liberated in Either Route is the Same San Francisco Chicago Denver QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Dallas New Haven Miami
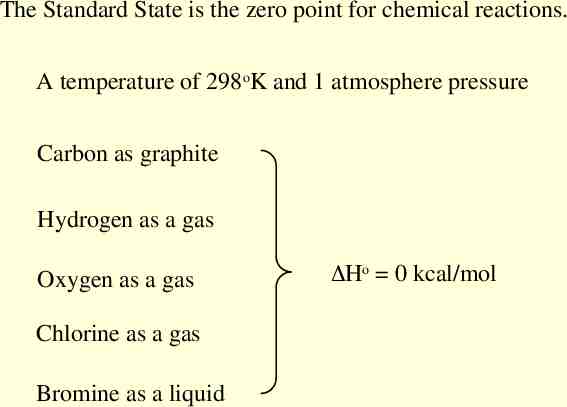
The Standard State is the zero point for chemical reactions. A temperature of 298oK and 1 atmosphere pressure Carbon as graphite Hydrogen as a gas Oxygen as a gas Chlorine as a gas Bromine as a liquid Ho 0 kcal/mol
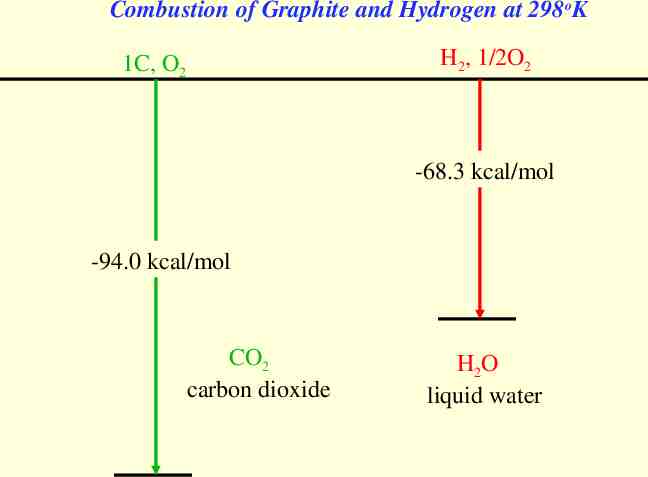
Combustion of Graphite and Hydrogen at 298oK H2, 1/2O2 1C, O2 -68.3 kcal/mol -94.0 kcal/mol CO2 carbon dioxide H2O liquid water
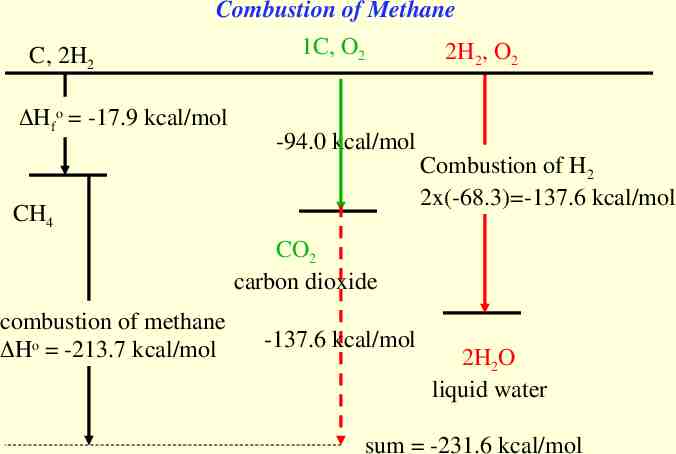
Combustion of Methane C, 2H2 Hfo -17.9 kcal/mol 1C, O2 2H2, O2 -94.0 kcal/mol Combustion of H2 2x(-68.3) -137.6 kcal/mol CH4 CO2 carbon dioxide combustion of methane Ho -213.7 kcal/mol -137.6 kcal/mol 2H2O liquid water sum -231.6 kcal/mol
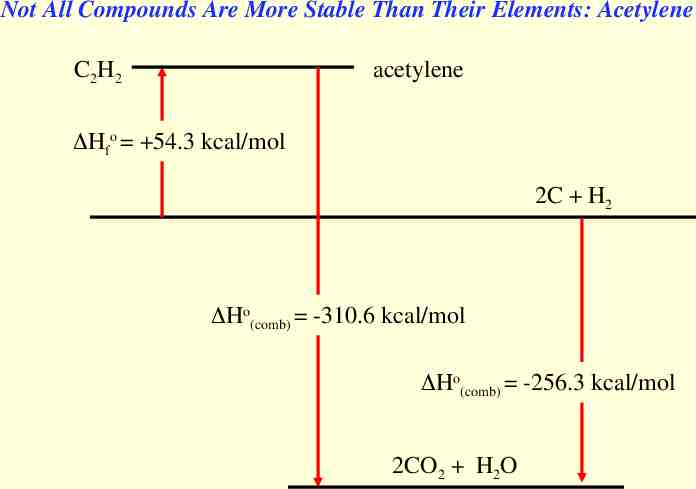
Not All Compounds Are More Stable Than Their Elements: Acetylene C2H2 acetylene Hfo 54.3 kcal/mol 2C H2 Ho(comb) -310.6 kcal/mol Ho(comb) -256.3 kcal/mol 2CO2 H2O
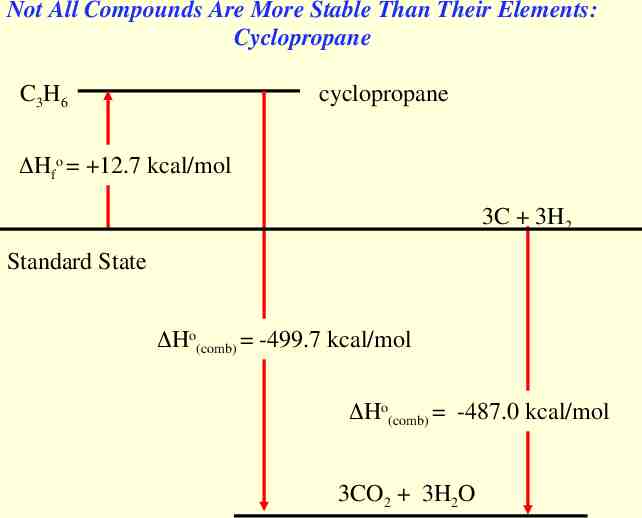
Not All Compounds Are More Stable Than Their Elements: Cyclopropane C3H6 cyclopropane Hfo 12.7 kcal/mol 3C 3H2 Standard State Ho(comb) -499.7 kcal/mol Ho(comb) -487.0 kcal/mol 3CO2 3H2O
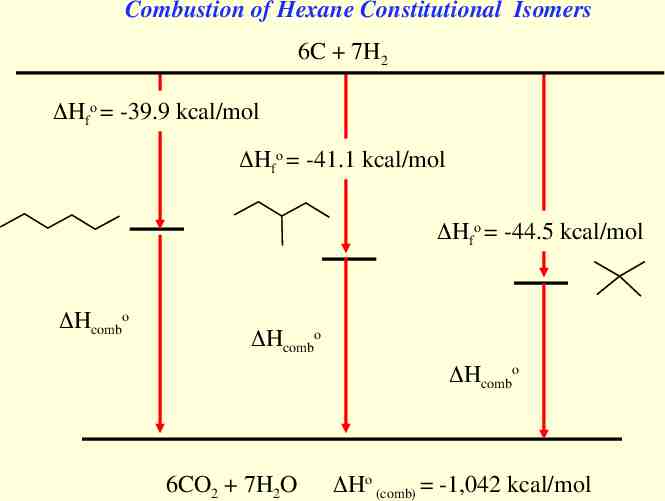
Combustion of Hexane Constitutional Isomers 6C 7H2 Hfo -39.9 kcal/mol Hfo -41.1 kcal/mol Hfo -44.5 kcal/mol Hcombo Hcombo Hcombo 6CO2 7H2O Ho (comb) -1,042 kcal/mol
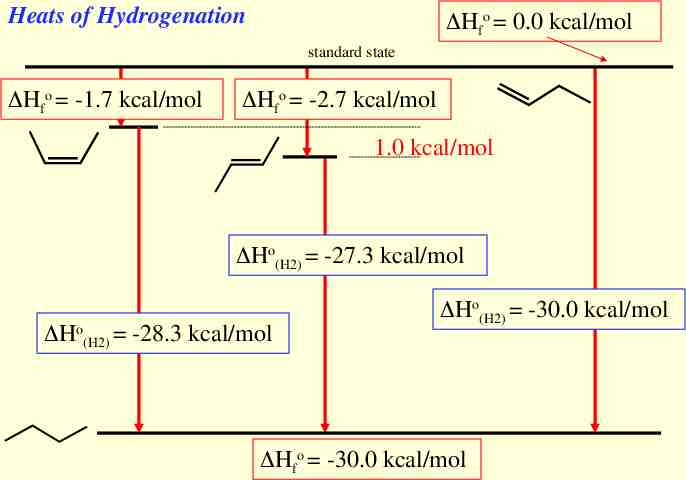
Heats of Hydrogenation Hfo 0.0 kcal/mol standard state Hfo -1.7 kcal/mol Hfo -2.7 kcal/mol 1.0 kcal/mol Ho(H2) -27.3 kcal/mol Ho(H2) -28.3 kcal/mol Ho(H2) -30.0 kcal/mol Hfo -30.0 kcal/mol
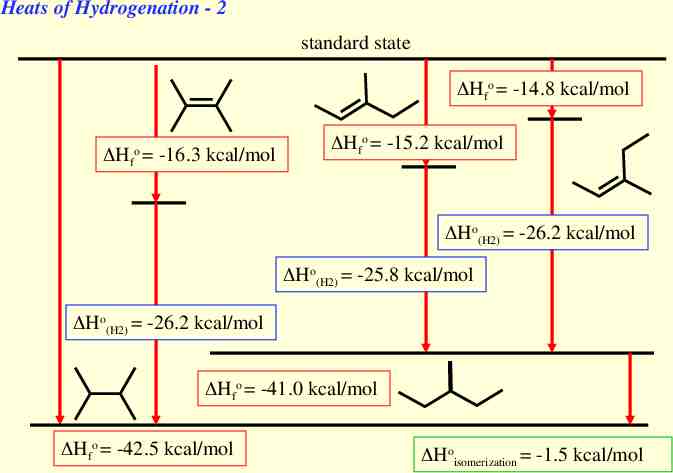
Heats of Hydrogenation - 2 standard state Hfo -14.8 kcal/mol o Hf -16.3 kcal/mol Hfo -15.2 kcal/mol Ho(H2) -26.2 kcal/mol Ho(H2) -25.8 kcal/mol Ho(H2) -26.2 kcal/mol Hfo -41.0 kcal/mol Hfo -42.5 kcal/mol Hoisomerization -1.5 kcal/mol
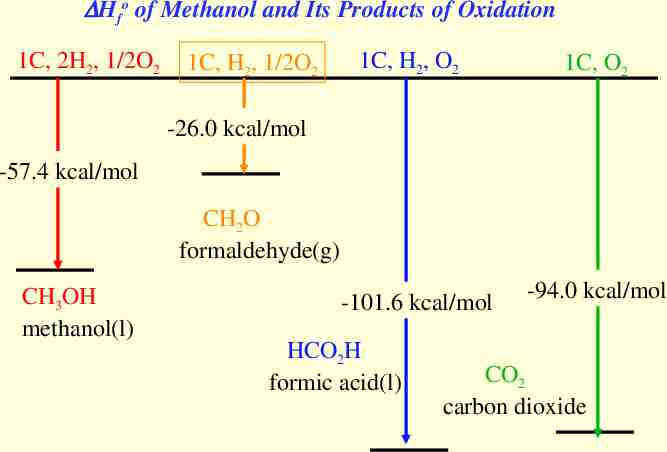
Hfo of Methanol and Its Products of Oxidation 1C, 2H2, 1/2O2 1C, H2, 1/2O2 1C, H2, O2 1C, O2 -26.0 kcal/mol -57.4 kcal/mol CH2O formaldehyde(g) CH3OH methanol(l) -101.6 kcal/mol HCO2H formic acid(l) -94.0 kcal/mol CO2 carbon dioxide
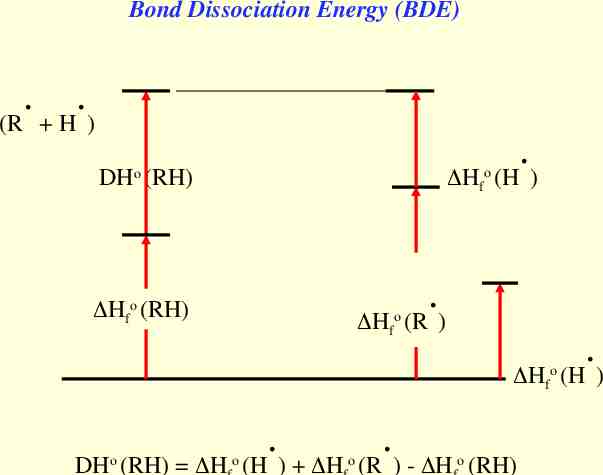
Bond Dissociation Energy (BDE) . . (R H ) . Hfo (H ) DHo (RH) . Hfo (RH) Hfo (R ) . Hfo (H ) . . DHo (RH) H o (H ) H o (R ) - H o (RH)
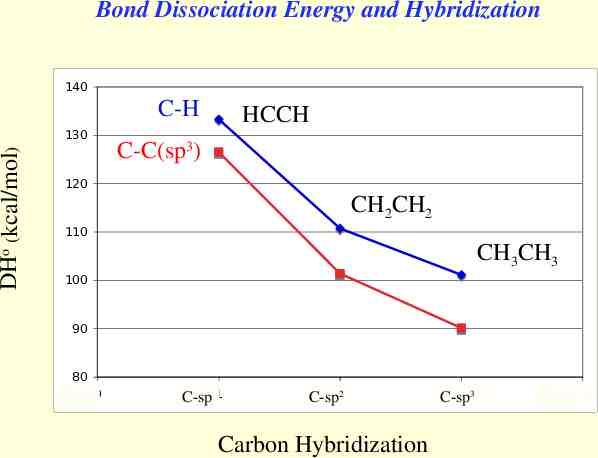
Bond Dissociation Energy and Hybridization 140 C-H DHo (kcal/mol) 130 HCCH C-C(sp3) 120 CH2CH2 110 CH3CH3 100 90 80 C-sp 0 C-sp 1 C-sp22 Carbon Hybridization 3 3 C-sp C-sp 4
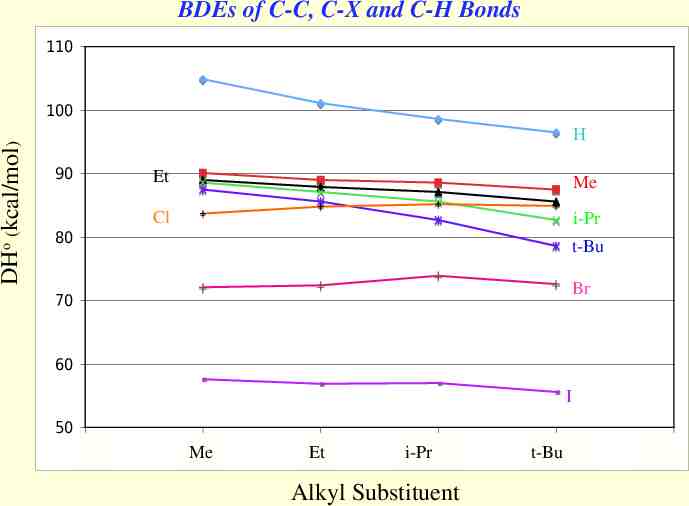
BDEs of C-C, C-X and C-H Bonds 110 DHo (kcal/mol) 100 H 90 80 Et Me Cl i-Pr t-Bu Br 70 60 I 50 0 Me 1 Me 2 Et Me i-Pr 3 Alkyl Substituent 4 t-Bu Me5
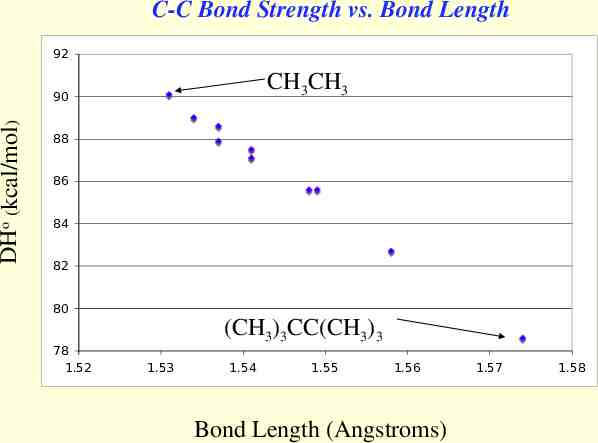
C-C Bond Strength vs. Bond Length 92 CH3CH3 DHo (kcal/mol) 90 88 86 84 82 80 78 1.52 (CH3)3CC(CH3)3 1.53 1.54 1.55 1.56 Bond Length (Angstroms) 1.57 1.58
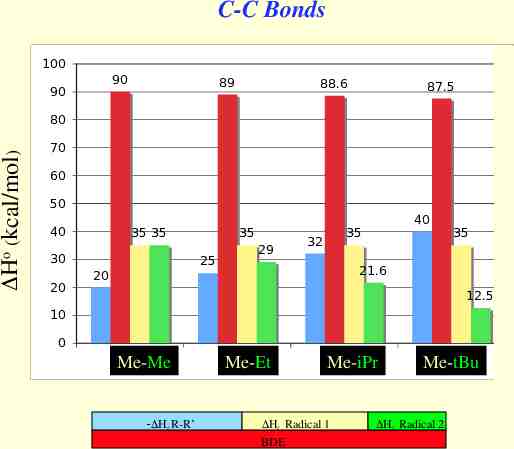
C-C Bonds 100 90 90 89 88.6 87.5 Ho (kcal/mol) 80 70 60 50 40 35 35 30 20 35 25 20 29 40 35 32 35 21.6 12.5 10 0 1 Me-Me Hf R-R’ 2 Me-Et 3 Me-iPr Hf Radical 1 BDE 4 Me-tBu Hf Radical 2 tBu-tBu
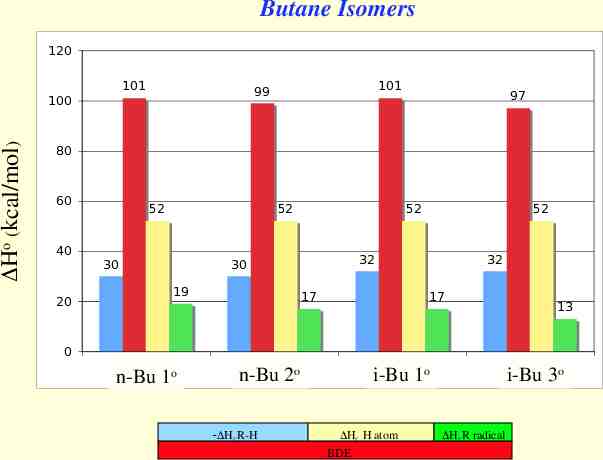
Butane Isomers 120 101 Ho (kcal/mol) 100 101 99 97 80 60 40 20 52 52 30 52 32 30 19 52 17 32 17 13 0 1 n-Bu 1o n-Bu2 2o Hf R-H 3 o i-Bu 1 Hf H atom BDE 4 i-Bu 3o Hf R radical
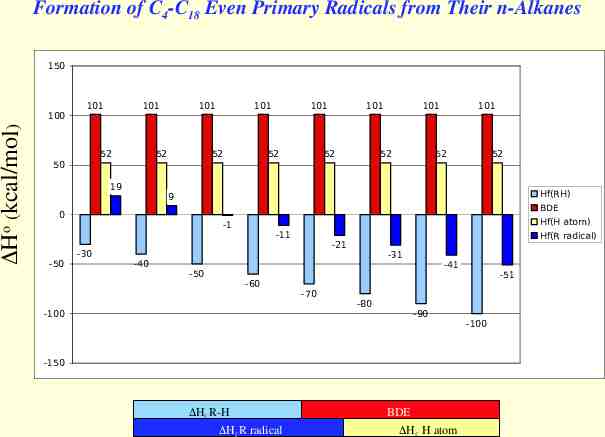
Formation of C4-C18 Even Primary Radicals from Their n-Alkanes 150 Ho (kcal/mol) 100 101 101 52 101 52 101 52 101 52 101 52 101 52 101 52 52 50 19 Hf(RH) 9 BDE 0 Hf(H atom) -1 -50 -30 -40 -50 -11 -60 -100 Hf(R radical) -21 -70 -31 -80 -41 -90 -150 Hf R-H Hf R radical BDE Hf H atom -51 -100

The End






