Gynecologic Oncology Group Protocol GOG-0258 Louise E. Francis,
20 Slides106.71 KB

Gynecologic Oncology Group Protocol GOG-0258 Louise E. Francis, B.S., CMD

Gynecologic Oncology Group (GOG) The only National Cancer Institute funded cooperative group in the US currently conducting trials 0n gynecologic cancers Has been conducting practice changing research for 40 years Design, conduct and monitor phase II & III trials involving cancers of the endometrium, uterine sarcomas and gestational trophoblastic neoplasia Most gynecologic oncologists participate

Background Historically for Stage III Endometrial Cancer, patients underwent surgery followed by radiation therapy (good local control) Systemic failure beyond treatment fields is an issue Chemotherapy for advanced endometrial cancer yielding good systemic control, but poor local control. The experimental arm of protocol GOG-0258 examines chemotherapy given concomitantly with radiation therapy. It is a randomized Phase III Protocol Study

GOG-0258 Objectives Primary Objective: To compare whether Cisplatin and volume directed radiation therapy, followed by carboplatin and paclitaxel for 4 cycles vs carboplatin and paclitaxel for 6 cycles better reduces the rate of recurrence or death in Stage III-IVa endometrial cancer patients. Secondary Objective: To assess acute and late treatment effects on Quality Of Life of patients before, during and after protocol treatment.
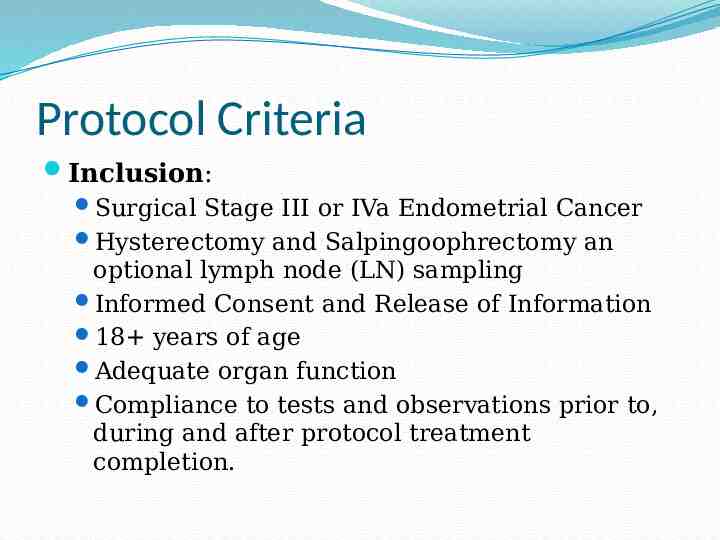
Protocol Criteria Inclusion: Surgical Stage III or IVa Endometrial Cancer Hysterectomy and Salpingoophrectomy an optional lymph node (LN) sampling Informed Consent and Release of Information 18 years of age Adequate organ function Compliance to tests and observations prior to, during and after protocol treatment completion.
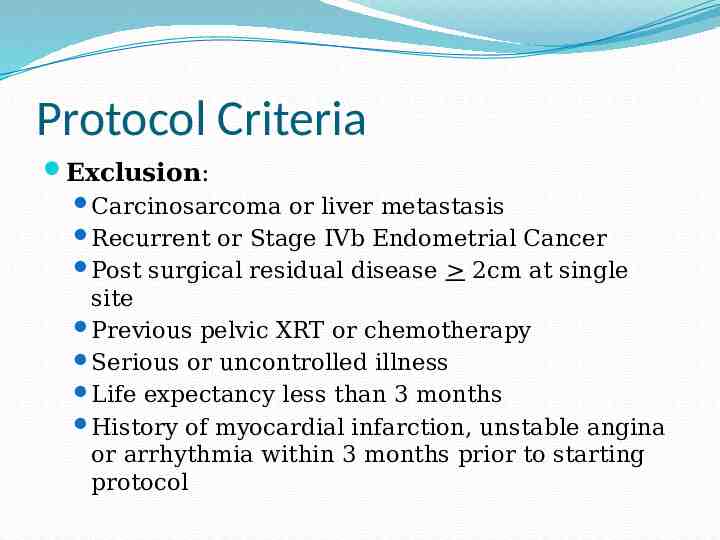
Protocol Criteria Exclusion: Carcinosarcoma or liver metastasis Recurrent or Stage IVb Endometrial Cancer Post surgical residual disease 2cm at single site Previous pelvic XRT or chemotherapy Serious or uncontrolled illness Life expectancy less than 3 months History of myocardial infarction, unstable angina or arrhythmia within 3 months prior to starting protocol
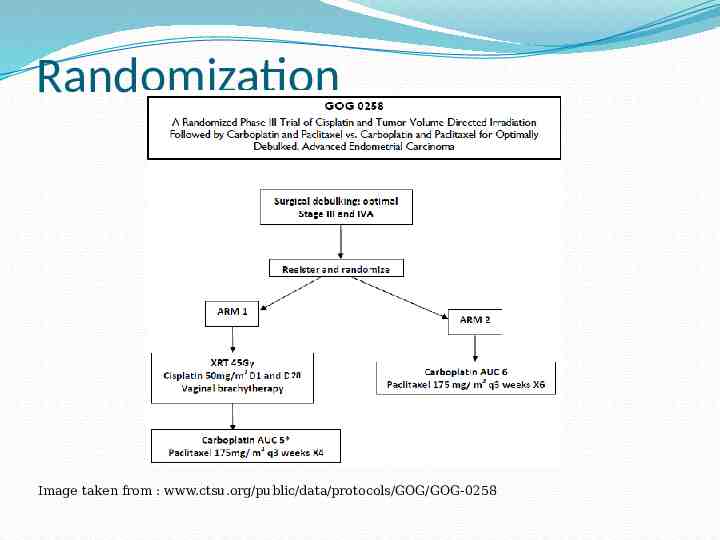
Randomization Image taken from : www.ctsu.org/public/data/protocols/GOG/GOG-0258

Protocol Discontinuation Discontinuation: Ideally it is hoped that all enrolled patients will be able to complete protocol treatments. However, discontinuation may occur for the following reasons: Patient choose to withdraw for any reason Delay of treatment over 3 consecutive weeks because of toxicity Lack of compliance on patient’s behalf

GOG-0258 Arm 1 Initial XRT given for 28 days (Cisplatin Day 1 & Day 28) Whole Pelvis Dose: 45 Gy in 25 fractions at 1.8Gy per day Boost of 10-15 Gy in 5-8 fractions may be given at radiation oncologist’s discretion. Carboplatin/Paclitaxel for 4 cycles to follow XRT within 8 weeks of chemoradiation completion Boost of 10-15Gy may be 3D conformal, IMRT, HDR or LDR depending on location of disease

GOG-0258 Arm 2 This arm of protocol involves chemotherapy only and represents the current standard of treatment for surgical Stage III and IVa endometrial cancer Chemothrapeutic agents Carboplatin plus Paclitaxel, are given every 21 days for 6 cycles Patients are observed carefully during this period for protocol related toxicity issues that may lead patient to withdrawal from protocol
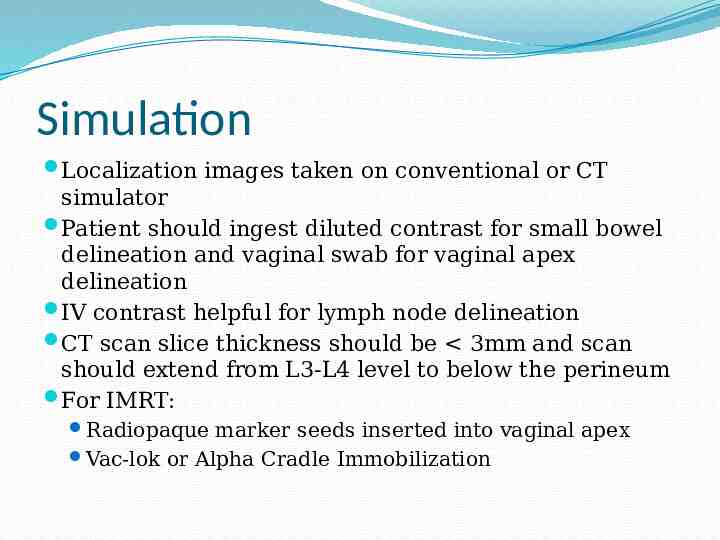
Simulation Localization images taken on conventional or CT simulator Patient should ingest diluted contrast for small bowel delineation and vaginal swab for vaginal apex delineation IV contrast helpful for lymph node delineation CT scan slice thickness should be 3mm and scan should extend from L3-L4 level to below the perineum For IMRT: Radiopaque marker seeds inserted into vaginal apex Vac-lok or Alpha Cradle Immobilization

Treatment Plan All radiation treatments must use 6-25 MV photons 3-D Conventional (4 Field Box): AP/PA Field Borders: Superior- L5-S1 interspace Inferior- Mid portion of obtorator foramen Lateral- 1cm widest portion of true pelvis Rt /Lt Lateral Field Borders: Same superior and inferior borders as AP/PA Anterior- At least 1.5 cm anterior to L5 Posterior- Bisect 3rd sacral vertebral body (3 cm margin on vaginal stump)

Treatment Plan Institutions utilizing IMRT treatments must be credentialed by the Radiologic Physics Center (RPC) at M.D. Anderson Cancer Center prior to entering a patient on protocol Credentialing involves irradiation of a standardized phantom from the RPC. The irradiated treatment plan must be electronically submitted to the Image-Guided Therapy Center for evaluation. The institution must await approval prior to proceeding with an IMRT plan

Treatment Plan Intensity Modulated Radiation Therapy (IMRT): In the event that IMRT is used for treatment, physicians must refer to the RTOG Gynecologic Atlas for volume specification when contouring. CTV must be contoured to include the vaginal apex with margin, pelvic lymph nodes and inguino femoral nodes if vaginal involvement is present PTV is 7 mm- 1 cm expansion of CTV in all directions
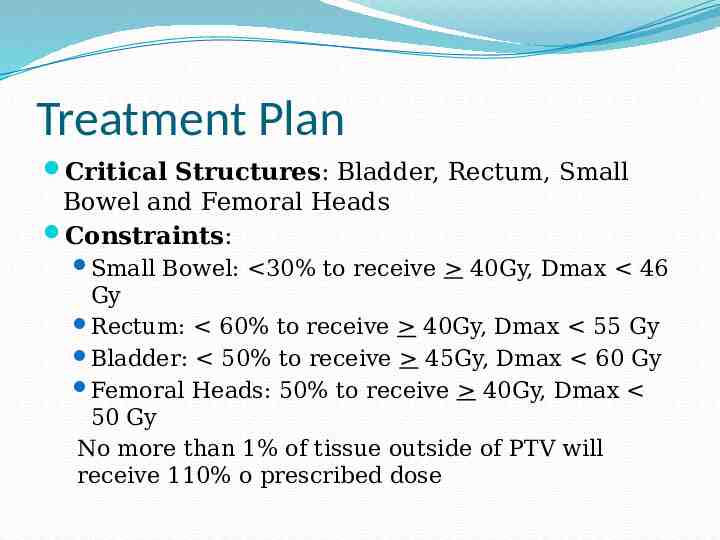
Treatment Plan Critical Structures: Bladder, Rectum, Small Bowel and Femoral Heads Constraints: Small Bowel: 30% to receive 40Gy, Dmax 46 Gy Rectum: 60% to receive 40Gy, Dmax 55 Gy Bladder: 50% to receive 45Gy, Dmax 60 Gy Femoral Heads: 50% to receive 40Gy, Dmax 50 Gy No more than 1% of tissue outside of PTV will receive 110% o prescribed dose

Expected Toxicities Radiation Therapy: Gastrointestinal symptoms may include nausea and vomiting. This more likely occur when Para-Aortic lymph nodes are treated Increased bowel activity with diarrhea can be expected after the two weeks of pelvic irradiation. Hematological toxicity of a mild nature will be seen with a decline in WBC and platelet count

Expected Toxicities Chemotherapy: Hematologic –myelosupression Gastrointestinal - nausea, vomiting, diarrhea, neutorpenia, colitis, ishemic colitis and mucositis Pulmonary – pneumonitis Heart – MI, arrhythmia, tachycardia and bradycardia Neurolgic- Sensory (taste),peripheral neuropathy, seizures, mood swings and encephopathy Liver – hepatic failure

Quality of Life Assessment Quality of life assessments will occur prior to, during and following treatment to asses physical and functional well being. They will occur in the following order Baseline: 14 days prior to treatment start 6 weeks from start of protocol 18 weeks from start of protocol 70 weeks from start of protocol

Conclusion . Clinical trials help to define future treatment regimens Patient and physician participation are vital to the success of all clinical trials To date, GOG-0258 has 298 of the 804 patients needed. It is due to close in December 2013 Thank You .

References 1. Mahtani, R. Treatment of Stage IV Endometrial Cancer. Cancer4Caring Web site. http:// www.caring4cancer.com/go/endometrial/treatments/treatm ent-of-stage-iv-endometrial-cancer.htm. Updated August 15, 2010. Accessed October 25, 2011. 2. Endometrial Cancer. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/types/endometrial. .Accessed October 23, 2011. 3. CTSU Protocols. Cancer Trials Support Unit Web site. https:// www.ctsu.org/public/prot search.aspx?browse cancer typ e&DiseaseId 7. Accessed October 28, 2011. 4. Protocol GOG-0258. Gynecologic Oncology Group Web site. http://www.gog.org/index.html.Accessed October 15, 2011.






