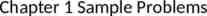Deborah A. Lichtenberg, RN, BSN, CIC Infection Preventionist Bard
59 Slides5.92 MB

Deborah A. Lichtenberg, RN, BSN, CIC Infection Preventionist Bard Medical 1

I am an employee of C. R. Bard, Inc., Bard Medical. Any discussion regarding Bard products during my presentation is limited to information that is consistent with Bard labeling for those products. 2

Describe the impact of CAUTIs on patient outcomes and hospital costs. Explain the pathogenesis of CAUTIs including the role of biofilm. Identify 4 changes/updates impact CAUTI surveillance and prevention. Review changes in practice identified above and the role of the hospital ICP. 3

Urinary tract infections are the most common type of healthcare-associated infection, accounting for more than 30% of infections reported by acute care hospitals. Virtually all healthcare-associated UTIs are caused by instrumentation of the urinary tract. Between 15% and 25% of hospitalized patients may receive short-term indwelling urinary catheters. Reported rates of UTI among patients with urinary catheters vary substantially. National data from NHSN acute care hospitals in 2006 showed a range of pooled mean CAUTI rates of 3.1-7.5 infections per 1000 catheter-days. The highest rates were in burn ICUs, followed by inpatient medical wards and neurosurgical ICUs The lowest rates were in medical/surgical ICUs. 4
Average cost of each uncomplicated UTI in 1992 was reported at 6804 - Based on total of 84 patients Average cost impact of each UTI reported in 1996 was 3,8035 - Based on 675 cases, 5,337 controls 5

At time of insertion Mechanical during catheter insertion the catheter picks up organisms urethral trauma during insertion Blockage of periurethral glands 6

Extraluminal – – – – Biofilm Encrustation Organisms migration Fecal incontinence Intraluminal – – – – Disconnection of catheter/drainage system Contamination of outlet tube Encrustation Biofilm 7
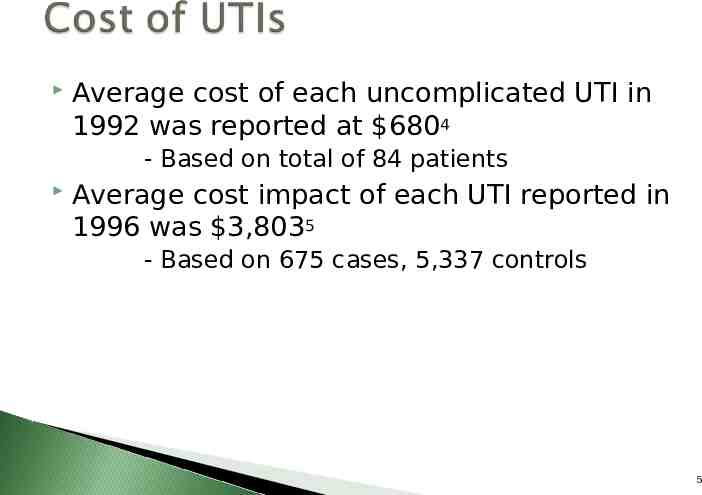
3 “Ports of Entry” Catheter / Meatal Junction Catheter / Tube Junction Outlet Tube 8
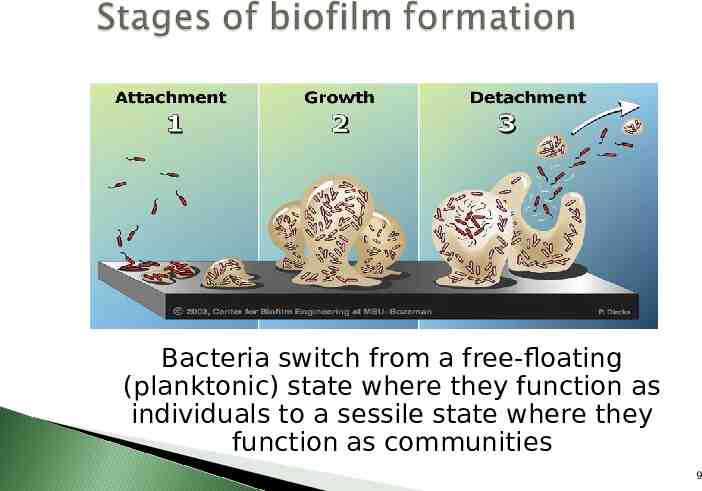
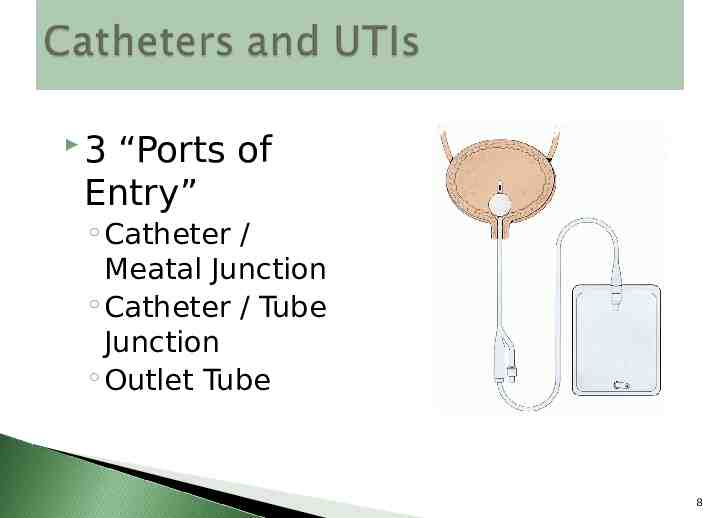
Bacteria switch from a free-floating (planktonic) state where they function as individuals to a sessile state where they function as communities 9
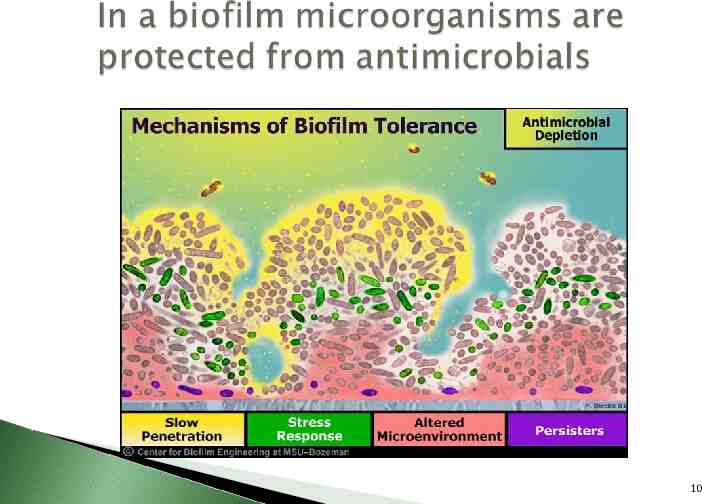
10

Catheter coatings are available to reduce bacterial adherence and prevent biofilm formation Silver – Bardex I.C. Anti-Infective Foley Catheter* – Dover Silver Foley Catheter – Silvertouch Foley Catheter Nitrofurazone – Release-NF Anti-Infective Foley Catheter 11
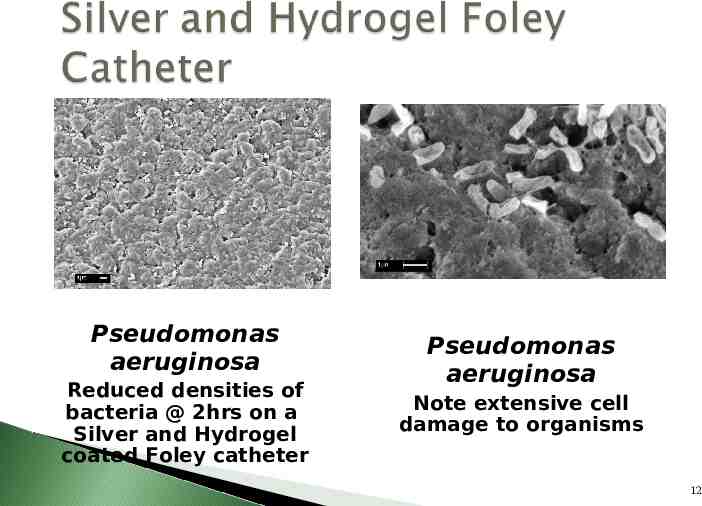
Pseudomonas aeruginosa Reduced densities of bacteria @ 2hrs on a Silver and Hydrogel coated Foley catheter Pseudomonas aeruginosa Note extensive cell damage to organisms 12

13

It all begins with Awareness – What is the clinical impact of CAUTI ? 9 UTIs account for 40% of all HAIs and of these, 80% are associated with urinary catheterization. – What CAUTI lack in terms of severity they make up with in terms of volume UTIs are the second most common cause of bloodstream infections and due to their frequency and subsequent treatment they are one of the largest breeding grounds for antibiotic resistant organisms – What is the financial impact of CAUTI ?9 UTIs cost U.S. hospitals more than 500 million per year to treat and can increase a patient’s length of stay by 3.8 days – Cost to Treat – Additional length of stay – Loss of CMS reimbursement 14
15

Changes in Reimbursement for Healthcare Acquired CAUTI 16

Hospitals will not receive additional payment for cases where the condition was not present upon admission Blood incompatibility Air embolism Object left after surgery Mediastinitis after CABG surgery Injuries from falls Vascular catheter associated infection Pressure ulcers Catheter associated urinary tract infection (CAUTI) 17
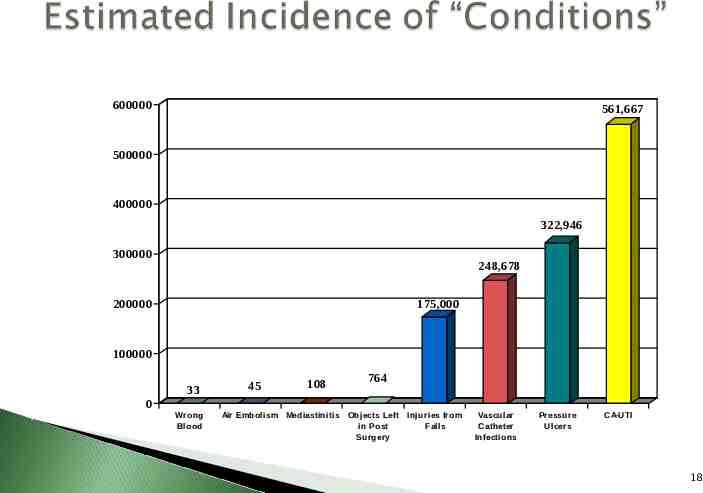
600000 561,667 500000 400000 322,946 300000 248,678 200000 175,000 100000 33 0 Wrong Blood 45 108 Air Embolism Mediastinitis 764 Objects Left in Post Surgery Injuries from Falls Vascular Catheter Infections Pressure Ulcers CA-UTI 18

High Volume: CDC reports there are 561,667 CAUTI every year – Most common healthcare-associated infection High Cost: APIC HAI Cost Calculator estimates cost to treat urinary tract infection 1,006 plus 6.3 days excess length of stay Assignment to Higher Paying DRG: Code 996.64 Reasonably Preventable: Prevention guidelines exist 19
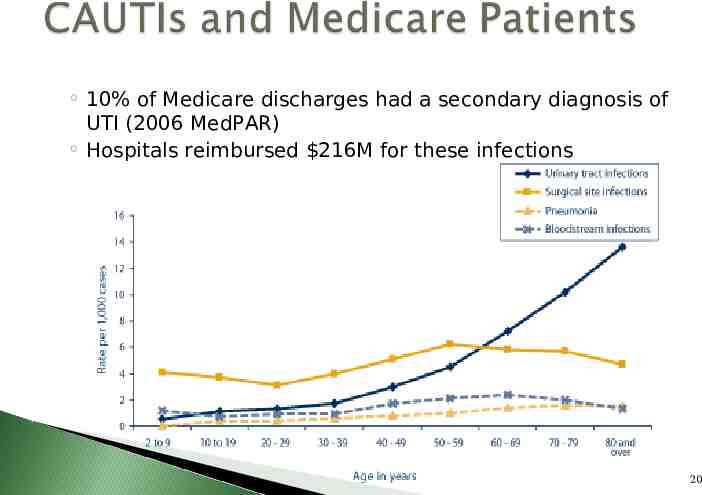
10% of Medicare discharges had a secondary diagnosis of UTI (2006 MedPAR) Hospitals reimbursed 216M for these infections 20

Medicare believes this will provide hospitals with additional incentive to engage in quality improvement efforts such as HAI reduction measures Presently they are developing a ValueBased Purchasing Rule (VBP) based on criteria from the Patient Protection and Affordable Care Act (ACA) 2010 21

Change in NHSN/CDC Definition for CA-UTIs 7 New HICPAC/CDC Guidelines for UTI Prevention9 Surgical Care Improvement Project (SCIP) 10 APIC Guide to Elimination of CatheterAssociated Urinary Tract Infections (CAUTIs) 11 22

UTI Definition for Patients with an Indwelling Foley Catheter7 23

# of symptomatic UTI / 1,000 urinary catheter days as measured in NHSN National 5-Year Prevention Target: 25% decrease from baseline Appendix G in HHS plan discusses a new type of metric, the standardized infection ratio (SIR) http://www.hhs.gov/ophs/initiatives/hai/prevtarg ets.html http://www.hhs.gov/ophs/initiatives/hai/appendic es.html
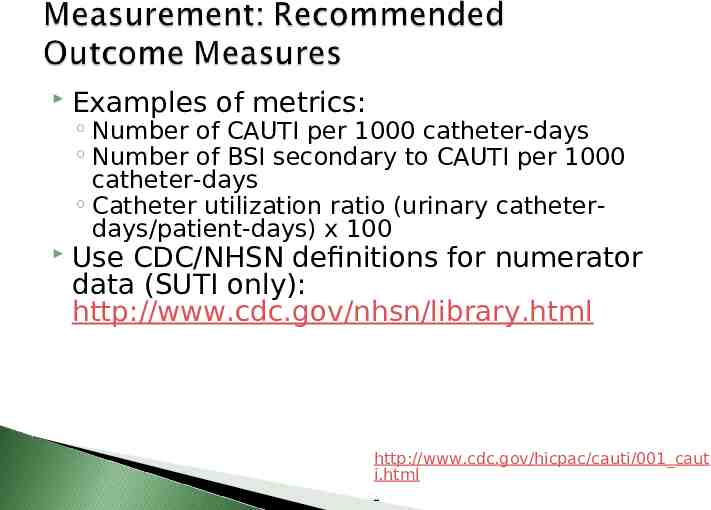
Examples of metrics: Number of CAUTI per 1000 catheter-days Number of BSI secondary to CAUTI per 1000 catheter-days Catheter utilization ratio (urinary catheterdays/patient-days) x 100 Use CDC/NHSN definitions for numerator data (SUTI only): http://www.cdc.gov/nhsn/library.html http://www.cdc.gov/hicpac/cauti/001 caut i.html

Symptomatic Infection Do catheterized patients have symptoms? Asymptomatic Bacteriuria Is it or is it not just colonization? 26
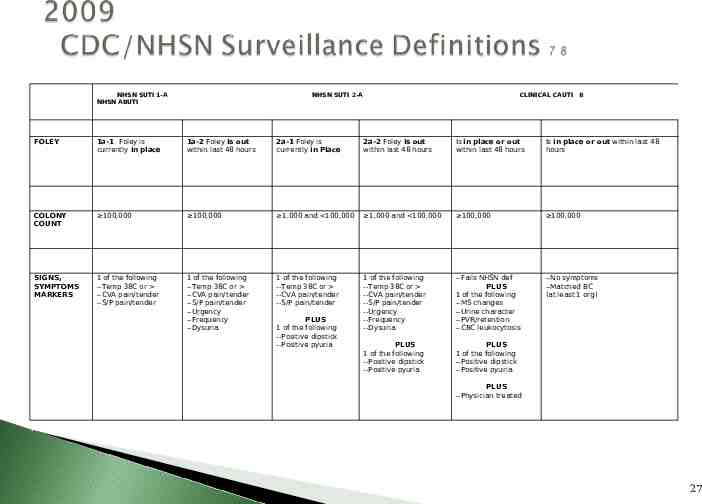
NHSN SUTI 1-A NHSN ABUTI NHSN SUTI 2-A CLINICAL CAUTI 8 FOLEY 1a-1 Foley is currently in place 1a-2 Foley is out within last 48 hours 2a-1 Foley is currently in Place 2a-2 Foley is out within last 48 hours Is in place or out within last 48 hours Is in place or out within last 48 hours COLONY COUNT 100,000 100,000 1,000 and 100,000 1,000 and 100,000 100,000 100,000 SIGNS, SYMPTOMS MARKERS 1 of the following --Temp 38C or --CVA pain/tender --S/P pain/tender 1 of the following --Temp 38C or --CVA pain/tender --S/P pain/tender --Urgency --Frequency --Dysuria 1 of the following --Temp 38C or --CVA pain/tender --S/P pain/tender 1 of the following --Temp 38C or --CVA pain/tender --S/P pain/tender --Urgency --Frequency --Dysuria --Fails NHSN def PLUS 1 of the following --MS changes --Urine character --PVR/retention --CBC leukocytosis --No symptoms --Matched BC (at least 1 org) PLUS 1 of the following --Positive dipstick --Positive pyuria PLUS 1 of the following --Positive dipstick --Positive pyuria PLUS 1 of the following --Positive dipstick --Positive pyuria PLUS --Physician treated 27

What are clinically relevant infections?8 Clinical indicators Physician diagnosis/treatment 8 McGeer A., et al. Definitions of Infections for Surveillance in Long Term Care Facilities, Am J Infect Control 1991; 19(1); 1-7. 28

Examples of programs that have been demonstrated to be effective include: A system of alerts or reminders to identify all patients with urinary catheters and assess the need for continued catheterization Guidelines and protocols for nurse-directed removal of unnecessary urinary catheters Education and performance feedback regarding appropriate use, hand hygiene, and catheter care Guidelines and algorithms for appropriate peri-operative catheter management, such as: Procedure-specific guidelines for catheter placement and postoperative catheter removal Protocols for management of postoperative urinary retention, such as nurse-directed use of intermittent catheterization and use of ultrasound bladder scanners 29

Although there have been several articles related to decreasing catheter usage, not all of these studies measured CAUTI as an outcome – At urinary catheter removal, 51 participants (19%) in the stop-order group developed urinary tract infection compared with 51 (20%) in the usual care group, relative risk 0.94, (95% CI, 0.66 to 1.33), P 0.71 – At 7 days post catheterization, 28 of those tested (21.1%) in the stop-order group compared to 19 (16.7%) in the usual care group had urinary tract infections, relative risk 1.26 (95% CI, 0.75 to 2.14), P 0.38. Study demonstrated that Foley catheter stop orders safely reduced Foley catheter usage but failed to reduce CAUTI 30
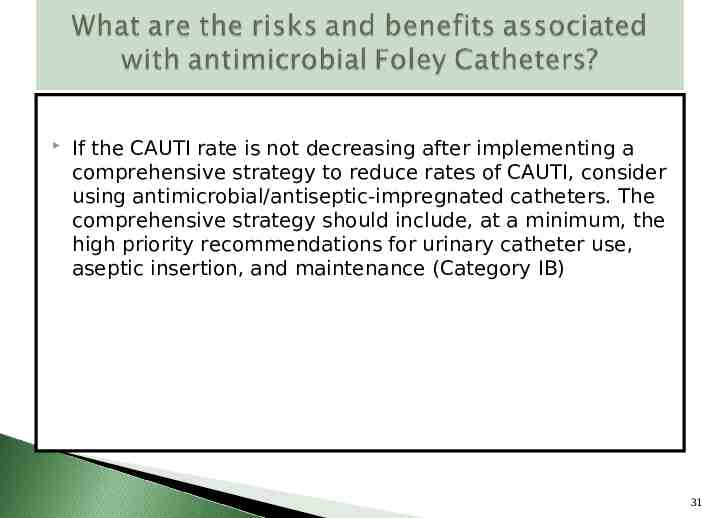
If the CAUTI rate is not decreasing after implementing a comprehensive strategy to reduce rates of CAUTI, consider using antimicrobial/antiseptic-impregnated catheters. The comprehensive strategy should include, at a minimum, the high priority recommendations for urinary catheter use, aseptic insertion, and maintenance (Category IB) 31

CAUTI Prevention Techniques – Appropriate Foley catheter Utilization – Proper Foley catheter Insertion, Maintenance, Removal – Monitoring Compliance – Continuing Education and Training CAUTI Prevention Technology – Bladder Scanners – Antimicrobial Foley Catheters Its not about what type of CAUTI prevention method works best; its about using every available method to try and prevent every CAUTI 32

PURPOSE To provide evidence-based practice guidance for the prevention of Catheter Associated Urinary Tract Infection (CAUTI) in acute and long term settings. 33

“Although infection control measures are the mainstay approach for preventing devicerelated infection, adherence to such measures is often inconsistent. That is why infection control measures need to be complemented with truly protective technology.” - Rabih O. Darouiche, M.D. Taken From Medical Devices Pose Big Infection Threat Copyright 2009 by Virgo Publishing. By: By Michelle Beaver 34

The Surgical Care Improvement Project (SCIP) is a national quality partnership of organizations focused on improving surgical care by significantly reducing surgical complications. 10 The SCIP goal is to reduce the incidence of surgical complications nationally by 25 percent by the year 2010. 35

SCIP-Inf-9 Urinary catheter removed on Postoperative Day (POD) 1or 2. 3 data elements added Urinary Catheter Catheter removal Reasons for continuing urinary catheterization 36
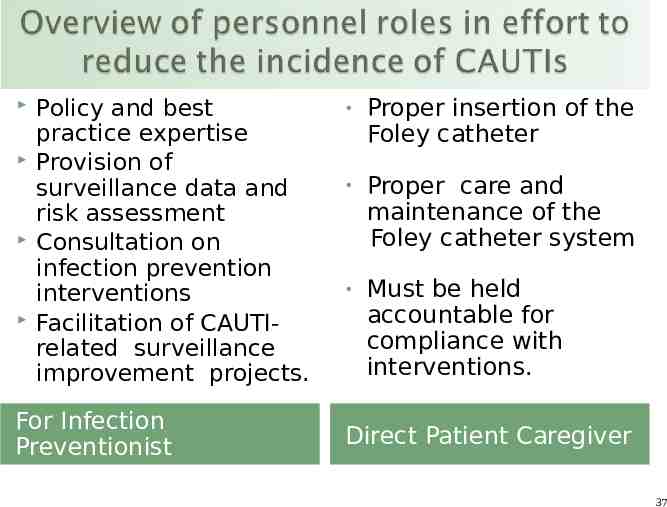
Policy and best practice expertise Provision of surveillance data and risk assessment Consultation on infection prevention interventions Facilitation of CAUTIrelated surveillance improvement projects. For Infection Preventionist Proper insertion of the Foley catheter Proper care and maintenance of the Foley catheter system Must be held accountable for compliance with interventions. Direct Patient Caregiver 37

Purpose To develop a surveillance, prevention and control plan based on facility specific data and conditions 38

Assess whether an effective organizational program exists. Assess population at risk Point Prevalence Survey Assess baseline outcome data Determine financial impact 39
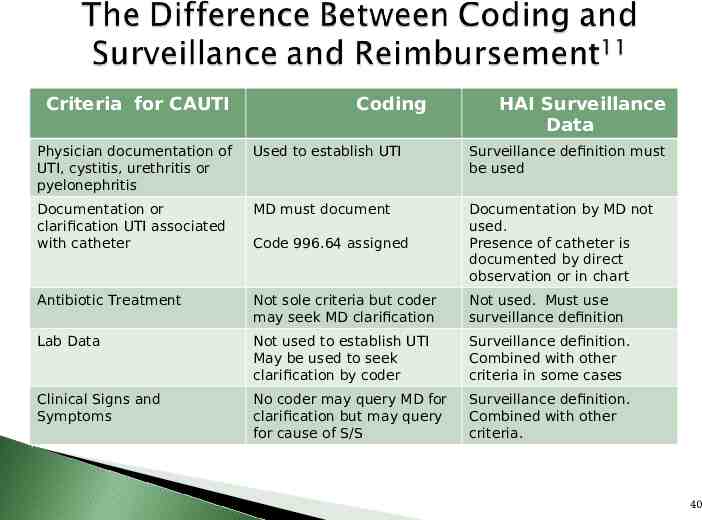
Criteria for CAUTI Coding HAI Surveillance Data Physician documentation of UTI, cystitis, urethritis or pyelonephritis Used to establish UTI Surveillance definition must be used Documentation or clarification UTI associated with catheter MD must document Code 996.64 assigned Documentation by MD not used. Presence of catheter is documented by direct observation or in chart Antibiotic Treatment Not sole criteria but coder may seek MD clarification Not used. Must use surveillance definition Lab Data Not used to establish UTI May be used to seek clarification by coder Surveillance definition. Combined with other criteria in some cases Clinical Signs and Symptoms No coder may query MD for clarification but may query for cause of S/S Surveillance definition. Combined with other criteria. 40

Assess need for Foley on a daily basis Implement early removal processes – Physician reminder systems – Nurse driven protocols – Automatic stop orders Early Foley removal for the surgical patient Consider routine use of bladder scanners Consider technology as addition to the comprehensive prevention plan 41

Aseptic insertion and maintenance Bladder ultrasound may avoid indwelling catherization Condom or intermittent catherization in appropriate patients Do not use the indwelling catheter unless you must. Early removal of the catheter using reminders or stop orders. 42

Are CAUTIs a Target for Improvement? Change in CDC Definition/Reporting to NHSN Changes in CMS Reimbursement Guidelines SHEA Compendium HICPAC/CDC SCIP APIC Guide to Elimination of CAUTIs 43

CAUTIs Are Important CAUTIs Have Serious Clinical and Economic Consequences Actions Can be Taken to Reduce CAUTIs 44

QUESTIONS? 45

1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Weinstein RA. Nosocomial Infection Update. Emerging Infectious Diseases. 1998; 4(3): 416-420. Salgado CD, Karchmer TB, and Farr BM. Prevention of Catheter-Associated Urinary Tract Infection. In Prevention and Control of Nosocomial Infections, 4thEd. Wenzel RP Ed. Philadelphia: Lippincott, Williams, and Wilkins, 2003. Saint S and Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin N Am. 2003; 17:411-432. Public health focus: surveillance, prevention and control of nosocomial infections. MMWR Morb Mortal Wkly Rep. 1992; 41:783-787. Classen D. Assessing the effect of adverse hospital events on the cost of hospitalization and other patient outcomes. University of Utah, 1993. SHEA Compendium, Strategies to Prevent Catheter Associated Urinary Tract Infections in Acute Care Hospitals. Infect Control Hospital Epidemiol 2008; 29: S41-S0. National Healthcare Safety Network (NHSN) Manual, March 2009. McGeer A., et al. Definitions of Infections for Surveillance in Long Term Care Facilities, Am J Infect Control 1991; 19(1); 1-7. HICPAC. Guideline for Prevention of Catheter-Associated Urinary Tract Infections; 2009. Surgical Care Improvement Project., 2010; Version 3.0a. APIC Guide to the Elimination of Catheter-Associated Urinary Tract Infections (CAUTIs), 2008. 46

www.APIC.org www.SHEA-online.org www.cfmc.org/hospital/hospital SCIP.html www.cdc.gov/ncidod/dhap/hicpac pub.html 47

Compliance with hand hygiene Compliance with educational program Compliance with documentation of catheter insertion and removal Compliance with documentation of indications for catheter placement http://www.cdc.gov/hicpac/cauti/001 caut i.html

Intermittent catheterization – consider for: Patients requiring chronic urinary drainage for neurogenic bladder Spinal cord injury Children with myelomeningocele Postoperative patients with urinary retention May be used in combination with bladder ultrasound scanners External (i.e., condom) catheters – consider for: Cooperative male patients without obstruction or urinary retention http://www.cdc.gov/hicpac/cauti/001 cauti.html

Consideration of alternatives to indwelling urinary catheterization (II) Use of portable ultrasound devices for assessing urine volume to reduce unnecessary catheterizations (II) Use of antimicrobial/antisepticimpregnated catheters (IB, after first implementing core recommendations for use, insertion, and maintenance ) The following slides will provide further details on supplemental strategies httpwww.cdc.gov/hicpac/cauti/001 cauti.html ://

Implement quality improvement programs to enhance appropriate use of indwelling catheters and reduce risk of CAUTI Examples: ―Alerts or reminders ―Stop orders ―Protocols for nurse-directed removal of unnecessary catheters ―Guidelines/algorithms for appropriate perioperative catheter management http://www.cdc.gov/hicpac/cauti/001 cauti.htm

Maintain unobstructed urine flow Keep catheter and collecting tube free from kinking Keep collecting bag below level of bladder at all times (do not rest bag on floor) Empty collecting bag regularly using a separate, clean container for each patient. Ensure drainage spigot does not contact nonsterile container. http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Following aseptic insertion, maintain a closed drainage system If breaks in aseptic technique, disconnection, or leakage occur, replace catheter and collecting system using aseptic technique and sterile equipment Consider systems with preconnected, sealed catheter-tubing junctions (II) Obtain urine samples aseptically http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Insert catheters using aseptic technique and sterile equipment (acute care setting) Perform hand hygiene before and after insertion Use sterile gloves, drape, sponges, antiseptic or sterile solution for periurethral cleaning, singleuse packet of lubricant jelly Properly secure catheters http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Insert catheters only for appropriate indications Minimize use in all patients, particularly those at higher risk of CAUTI and mortality (women, elderly, impaired immunity) Avoid use for management of incontinence Use catheters in operative patients only as necessary http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Insert catheters only for appropriate indications http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Insert catheters only for appropriate indications Leave catheters in place only as long as needed Ensure that only properly trained persons insert and maintain catheters Insert catheters using aseptic technique and sterile equipment (acute care setting) Following aseptic insertion, maintain a closed drainage system Maintain unobstructed urine flow Hand hygiene and Standard (or appropriate isolation) Precautions http://www.cdc.gov/hicpac/cauti/ 001 cauti.html

Core Strategies High levels of scientific evidence Demonstrated feasibility Supplemental Strategies Some scientific evidence Variable levels of feasibility *The Collaborative should at a minimum include core prevention strategies. Supplemental prevention strategies also may be used. Most core and supplemental strategies are based on HICPAC guidelines. Strategies that are not included in HICPAC guidelines will be noted by an asterisk (*) after the strategy. HICPAC guidelines may be found at www.cdc.gov/hicpac
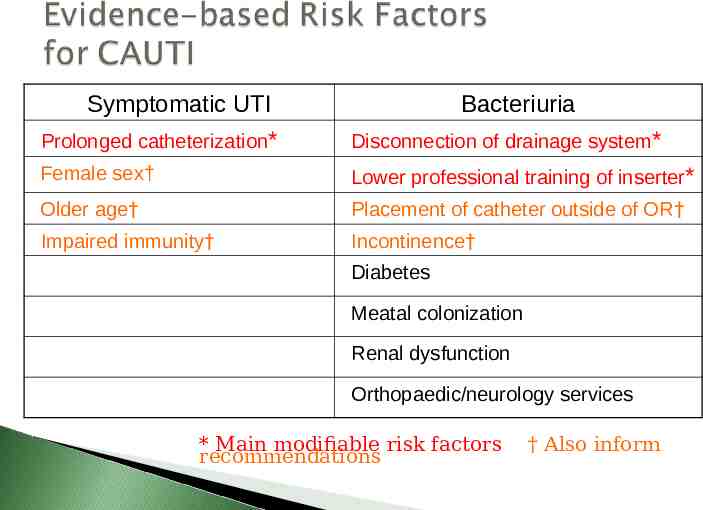
Symptomatic UTI Bacteriuria Prolonged catheterization* Disconnection of drainage system* Female sex† Lower professional training of inserter* Older age† Placement of catheter outside of OR† Impaired immunity† Incontinence† Diabetes Meatal colonization Renal dysfunction Orthopaedic/neurology services * Main modifiable risk factors recommendations † Also inform