Chemical Measurements Unit 1: Stoichiometry Chapter 10 – The Mole
16 Slides261.00 KB
Chemical Measurements Unit 1: Stoichiometry Chapter 10 – The Mole

Monday, 2/2 Learning Target: Distinguish between atomic mass, formula mass and molar mass. Learning Outcome: Complete “Formula Mass and Molar Mass” Worksheet.
Measurements in Chemistry We use measurements all of the time! Atomic Mass is a measurement that we have already used in class. o units for atomic mass atomic mass unit (amu) o Used to express masses of atoms on a relative scale. We compare everything to carbon-12.
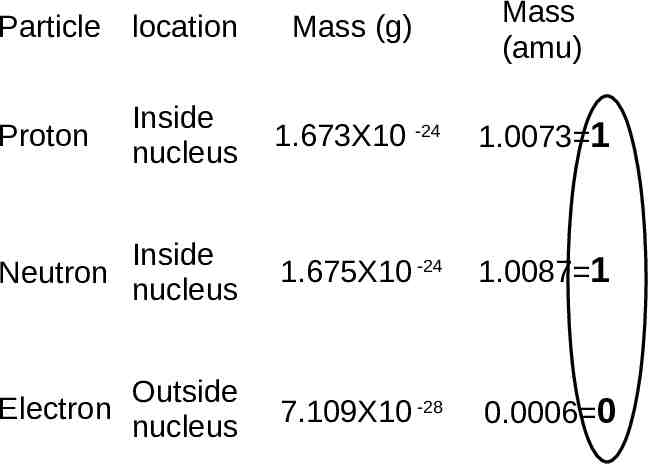
Mass (g) Mass (amu) Inside nucleus 1.673X10 -24 1.0073 1 Inside Neutron nucleus 1.675X10 -24 1.0087 1 Outside Electron nucleus 7.109X10 -28 0.0006 0 Particle location Proton

Atomic Mass & Formula Mass Atomic Mass The weighted average of the masses of the existing isotopes of an element o Ex. Carbon C 12.01 amu o Formula Mass The sum of the atomic masses of all atoms in a compound o Ex. Carbon Dioxide CO 44.01 amu 2 o

Calculating Formula Mass What is the formula mass for SO2? o o S: 1 atom x 32.1 amu O: 2 atoms x 16.0 amu Formula Mass 32.1 amu 32.0 amu 64.1 amu

Calculating Formula Mass What is the formula mass for H2O2? o o H: 2 atoms x 1.01 amu O: 2 atoms x 16.0 amu Formula Mass 2.02 amu 32.0 amu 34.02 amu

Calculating Formula Mass Methylene chloride (CH2Cl2) is used as a solvent in paint strippers. What is the formula mass of methylene chloride? o o o C: 1 atom x 12.01 amu H: 2 atoms x 1.01 amu Cl: 2 atoms x 35.45 amu Formula Mass 12.01(1) 1.01(2) 35.45(2) 85.0 amu

Moving from AMU to grams The use of atomic mass units (amu) are impractical in the chemistry lab where the preferred unit of measurement is grams. Scientists needed to establish a relationship between # of atoms and masses of atoms.
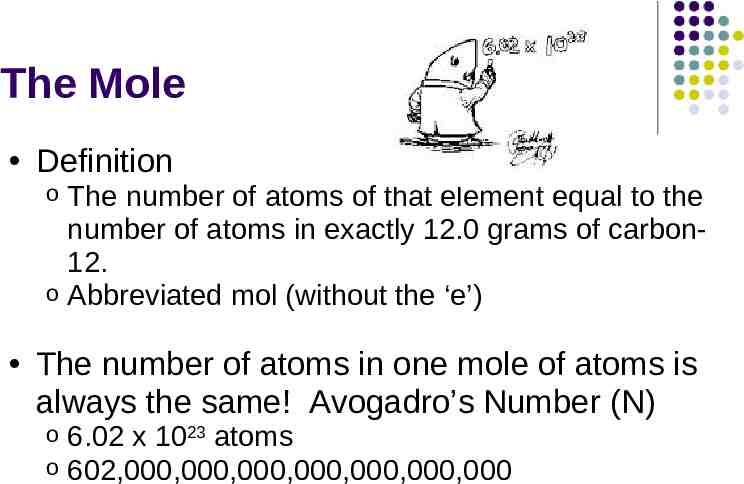
The Mole Definition The number of atoms of that element equal to the number of atoms in exactly 12.0 grams of carbon12. o Abbreviated mol (without the ‘e’) o The number of atoms in one mole of atoms is always the same! Avogadro’s Number (N) o o 6.02 x 1023 atoms 602,000,000,000,000,000,000,000

Same Number, Different Mass Element # atoms/mol mass of 1 mole Carbon 6.02x1023 atoms 12.01 g C Copper 6.02x1023 atoms 63.55 g Cu Tin 6.02x1023 atoms 118.71 g Sn Why do the amounts of each look different?

Calculating Molar Mass Methylene chloride (CH2Cl2) o o o C: 1 atom x 12.01 amu H: 2 atoms x 1.01 amu Cl: 2 atoms x 35.45 amu Formula Mass 12.0(1) 1.0(2) 35.5(2) 85.0 amu Molar Mass 84.95 g/mol
Molecules and Moles The number of molecules in 1mole of a molecular compound is 6.02 x 1023 (same as with atoms in a mole) 1 mol of water (H2O) contains 1 mol of water molecules but 2 mol of hydrogen atoms and 1 mol oxygen. How many moles of Ca2 and F- are in 1 mole of calcium fluoride? o 1 mole of Ca2 ions and 2 moles of F- ions

Molar Mass Definition o The mass in grams of 1 mole of a substance We calculate molar mass the same way as formula mass. o Atomic/Formula Molar Mass o Ex. Calcium 40.08 amu 40.08 g/mol

Same Number, Different Mass Practice What is the formula and molar mass for propane, C3H8? – C: 3 atoms x 12.01 amu 36.03 amu – H: 8 atoms x 1.01 amu 8.08 amu Formula Mass 36.03 amu 8.08 amu 44.11 amu Molar Mass 44.11 g/mol

Same Number, Different Mass Practice What is the formula and molar mass for glucose, C6H12O6? – C: 6 atoms x 12.01 amu 72.06 amu – H: 12 atoms x 1.01 amu 12.12 amu – O: 6 atoms x 16.00 amu 96.00 amu Formula Mass 72.06 amu 12.12amu 96 amu 180.18 amu Molar Mass 180.18 g/mol






