CHAPTER 6 TEST: CHEMICAL BONDING REVIEW SHEET
9 Slides62.00 KB

CHAPTER 6 TEST: CHEMICAL BONDING REVIEW SHEET

1. What are the 2 main types of bonds that we have learned about? 1. IONIC AND COVALENT 2. During an ionic bond, valence electrons are 2. TAKEN OR GIVEN AWAY 3. During a covalent bond, valence electrons are 3. SHARED 4. What periodic trend can be used to determine the type of bond that elements will form? 4. ELECTRONEGATIVITY

5. What levels of differences in electronegativity correspond to polar covalent, nonpolar covalent, and ionic bonds? 5. IONIC: 3.3-1.7, P.C.: 1.7-0.3, N.P.C.: 0.3-0.0 6. If Sodium, Na, was to bond with Chlorine, Cl, what type of bond would they form? 6. IONIC 7. If Bromine, Br was to bond with Chlorine, Cl, what type of bond would they form? 7. NON POLAR COVALENT 8. If Hydrogen, H was to bond with Oxygen, O, what type of bond would they form? 8. POLAR COVALENT

9. According to the octet rule, a calcium atom has a tendency to do what? 9. LOSE 2 VALENCE ELECTRONS 10. As the electronegativity difference between bonded atoms decreases, the bond becomes more Ionic or Covalent? 10. COVALENT 11. As atoms bond with each other, what happens to their potential energy? 11. DECREASE IN POTENTIAL ENERGY 12. As atoms bond with each other, do they become more or less stable? 12. MORE STABLE
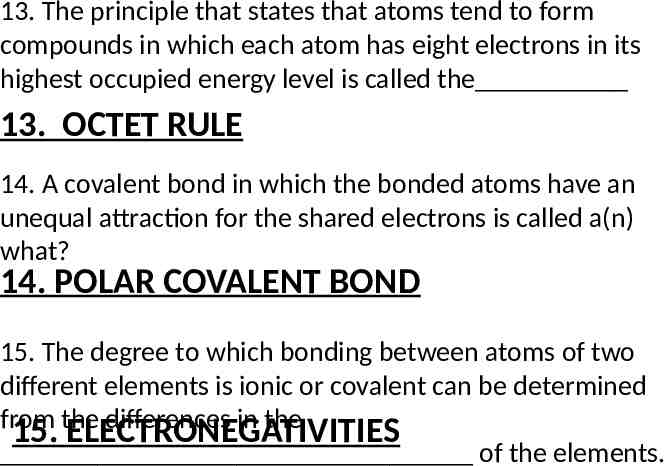
13. The principle that states that atoms tend to form compounds in which each atom has eight electrons in its highest occupied energy level is called the 13. OCTET RULE 14. A covalent bond in which the bonded atoms have an unequal attraction for the shared electrons is called a(n) what? 14. POLAR COVALENT BOND 15. The degree to which bonding between atoms of two different elements is ionic or covalent can be determined from the differences in the 15. ELECTRONEGATIVITIES of the elements.
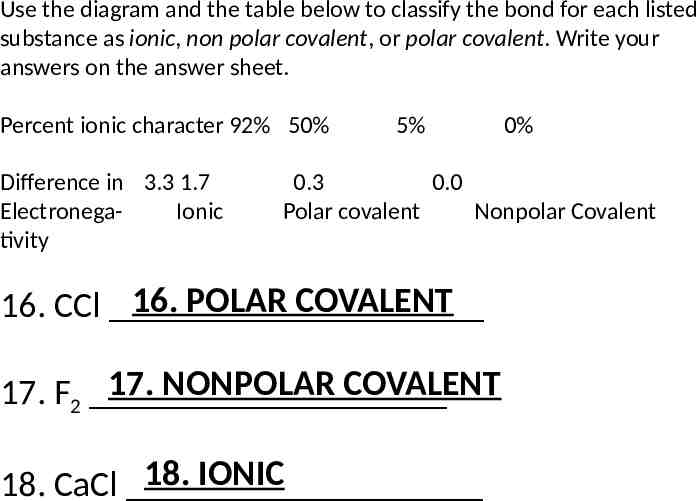
Use the diagram and the table below to classify the bond for each listed substance as ionic, non polar covalent, or polar covalent. Write your answers on the answer sheet. Percent ionic character 92% 50% Difference in 3.3 1.7 ElectronegaIonic tivity 5% 0% 0.3 0.0 Polar covalent Nonpolar Covalent 16. POLAR COVALENT 16. CCl 17. NONPOLAR COVALENT 17. F2 18. IONIC 18. CaCl
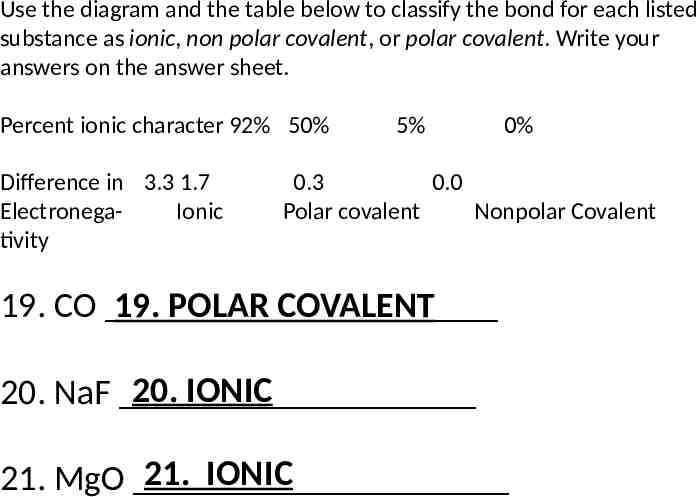
Use the diagram and the table below to classify the bond for each listed substance as ionic, non polar covalent, or polar covalent. Write your answers on the answer sheet. Percent ionic character 92% 50% Difference in 3.3 1.7 ElectronegaIonic tivity 5% 0% 0.3 0.0 Polar covalent Nonpolar Covalent 19. CO 19. POLAR COVALENT 20. IONIC 20. NaF 21. IONIC 21. MgO
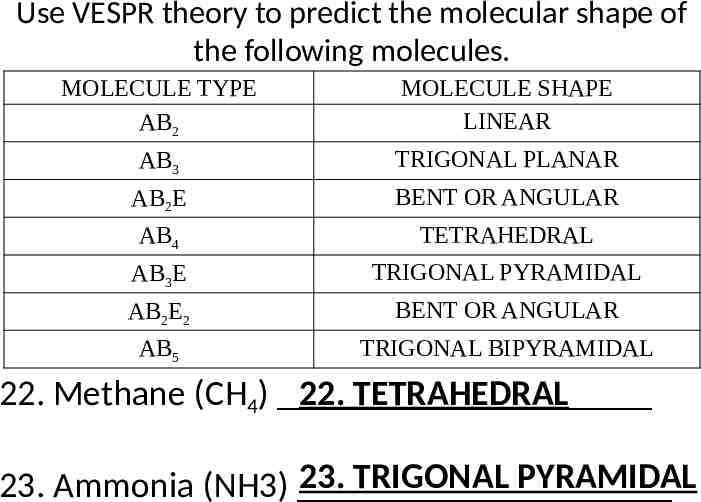
Use VESPR theory to predict the molecular shape of the following molecules. MOLECULE TYPE AB2 MOLECULE SHAPE LINEAR AB3 TRIGONAL PLANAR AB2E BENT OR ANGULAR AB4 TETRAHEDRAL AB3E TRIGONAL PYRAMIDAL AB2E2 BENT OR ANGULAR AB5 TRIGONAL BIPYRAMIDAL 22. Methane (CH4) 22. TETRAHEDRAL 23. TRIGONAL PYRAMIDAL 23. Ammonia (NH3)
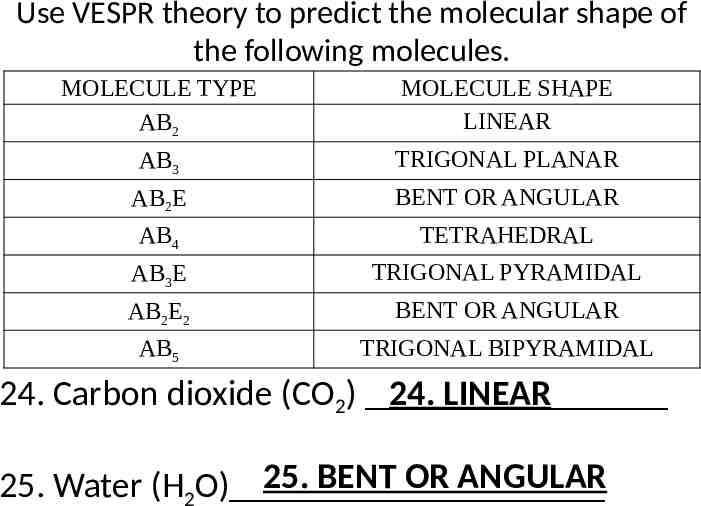
Use VESPR theory to predict the molecular shape of the following molecules. MOLECULE TYPE AB2 MOLECULE SHAPE LINEAR AB3 TRIGONAL PLANAR AB2E BENT OR ANGULAR AB4 TETRAHEDRAL AB3E TRIGONAL PYRAMIDAL AB2E2 BENT OR ANGULAR AB5 TRIGONAL BIPYRAMIDAL 24. Carbon dioxide (CO2) 24. LINEAR 25. BENT OR ANGULAR 25. Water (H2O)






