CEMENT Manufacture of Cement DRY PROCESS: In the Dry and Semi-dry
18 Slides1.09 MB

CEMENT Manufacture of Cement DRY PROCESS: In the Dry and Semi-dry processes, the raw materials are crushed and fed in correct proportions into a grinding mill They are dried and reduced in size to a fine powder. The dry powder called raw meal is then pumped into a blending silo, where an intimate and uniform mixture is obtained, usually by introducing a compressed air. In the Semi-dry process, the blended meal is sieved and fed into a rotating dish called granulator and water weighing about 12 % of the meal is added at the same time. In this manner hard pellets of about 15 mm in diameter are formed This is necessary, as cold powder fed direct into the kiln would not permit the air flow and exchange of heat necessary for chemical reaction required for the formation of cement clinker. The pellets are baked hard in a pre-heating grate by means of hot gases from the Kiln. The pellets then enter the kiln for burning and subsequent conversion into clinker. 1
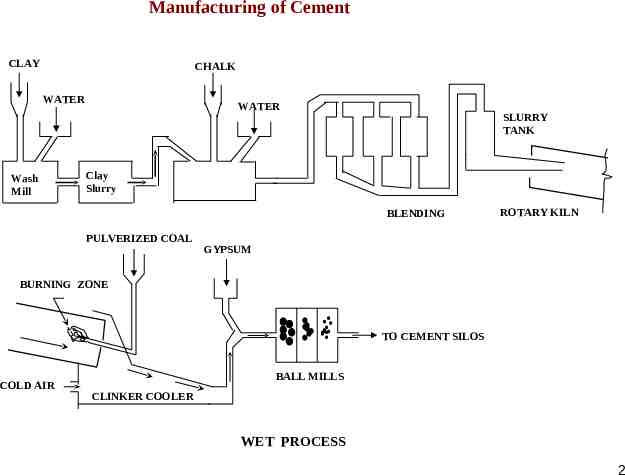
Manufacturing of Cement CLAY CHALK WATER Wash Mill WATER SLURRY TANK Clay Slurry BLENDING PULVERIZED COAL ROTARY KILN GYPSUM BURNING ZONE TO CEMENT SILOS COLD AIR BALL MILLS CLINKER COOLER WET PROCESS 2

Cross-section Multi Compartment Silo Electronic Packers: It has continuous weighing system & ensures that the bags separating from the nozzles have accurate weight of Cement Jumbo Bag Packing 3

Since the moisture content is only 12 % as compared to 40 % in the Wet Process, the size of the kiln is comparatively smaller The heat required is also much smaller, thereby making this process quit economical. The total consumption of Coal in this method is only 100 Kg whereas in the wet process the requirement of coal is 350 Kg for producing 1 ton of cement. CHEMICAL COMPOSITION OF PORTLAND CEMENT:- Following are the major constituents of cement Name of Compound Oxide Composition Abbreviation Tri-Calcium Silicate 3CaO SiO2 C3 S Di-Calcium Silicate 2CaO SiO2 C2 S Tri-Calcium Aluminate 3CaO Al2 O3 C3 A Tetra-Calcium Alumino ferrite 4CaO Al2 O3Fe2O3 [CaO – C & SiO2 – S] [Al2O3 – A] C4AF [Fe2O3 – F] The above compounds are also known as Bogue’s Compounds based on the work done by R. H. Bogue and others. 4
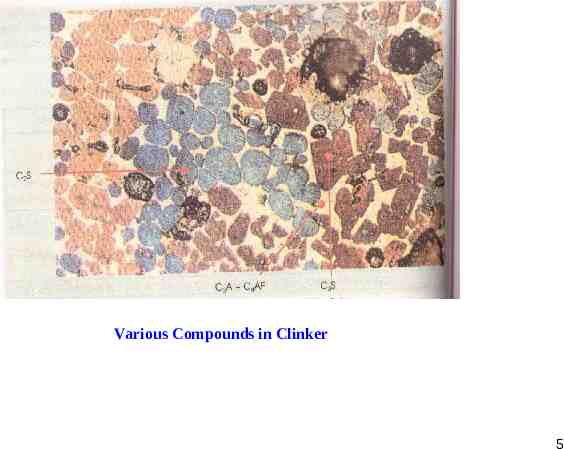
Various Compounds in Clinker 5
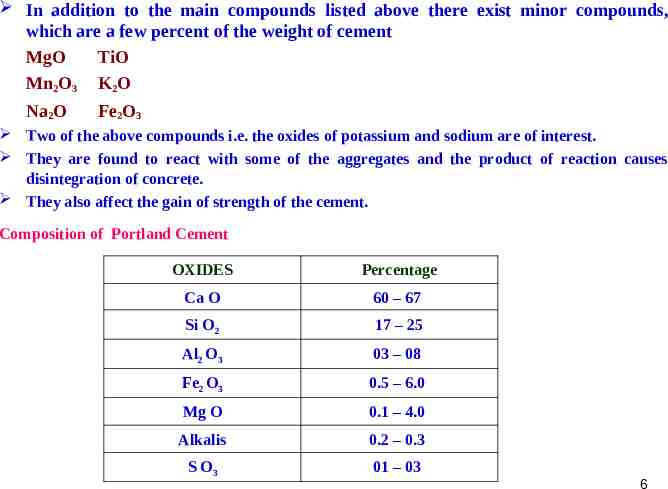
In addition to the main compounds listed above there exist minor compounds, which are a few percent of the weight of cement MgO TiO Mn2O3 K2O Na2O Fe2O3 Two of the above compounds i.e. the oxides of potassium and sodium are of interest. They are found to react with some of the aggregates and the product of reaction causes disintegration of concrete. They also affect the gain of strength of the cement. Composition of Portland Cement OXIDES Percentage Ca O 60 – 67 Si O2 17 – 25 Al2 O3 03 – 08 Fe2 O3 0.5 – 6.0 Mg O 0.1 – 4.0 Alkalis 0.2 – 0.3 S O3 01 – 03 6

The Silicates C3S and C2S are responsible for the strength of hydrated cement paste. The Aluminate C3A in cement paste is undesirable: as it contribute little or nothing to the strength of the cement except at early ages. However C3A is beneficial in the manufacture of cement as it facilitates the combination of lime and silica. The reaction by virtue of which Portland Cement becomes a bonding agent, takes place in water cement paste. In other words, in the presence of water, the silicates and aluminates forms the products of hydration, which in time produces a firm and hard mass – The Hardened Cement Paste. The Silicates C3S and C2S are the main cementitious compounds in cement. In commercial cements, the calcium silicate contains some small impurities from some of the oxides present in the clinker. These impurities have a strong effect on the properties of the hydrated cement. The impure C 3S is known as alite and the impure C2S as belite. C3S hydrates more rapidly than C2S. The product of hydration of C3S and C2S is the micro-crystalline hydrate C3S2H3 with some lime separating out as crystalline Ca(OH)2. C2S produces less lime than C3S. 7

Hydration of Cement Anhydrous Cement Compounds when mixed with water react with other to form Hydrated Compounds of very low solubility . The reaction of Cement with Water is Exothermic. The reaction liberates considerable amount of heat This liberation of heat is termed as heat of Hydration The Hydration Process is not an Instantaneous process – Faster in the early Period and Continues indefinitely at a decreasing rate. Complete Hydration cannot be obtained under a period of one year or so. The product of hydration shows unattacked Cores of Calcium Silicate, surrounded by a layer of Hydrated Silicate This layer of Hydrated Silicate is Impervious and further slows down the Hydration Reaction After 28 days of Curing, it is found that the Cement Grain is Hydrated only upto a depth of 4 . It is also observed that complete hydration is possible only for cement particle smaller than 50 The Largest Crystal of C3S or C2S is about 40 with an average size being 15-20 C2S present at the surface of the Cement Grain may get Hydrated and a more reactive C3S lying in the interior of the cement grain may not get hydrated 8

The Hydrated product adheres firmly to the unhydrated Core of Cement Grain Hence Unhydrated Cement left in a grain of cement will not reduce the strength of cement mortar or concrete as long as the product of hydration are well compacted. Nowadays Calcium Silicate hydrates are described as C-S-H gel. ( Previously referred to as tobermorite gel) 9
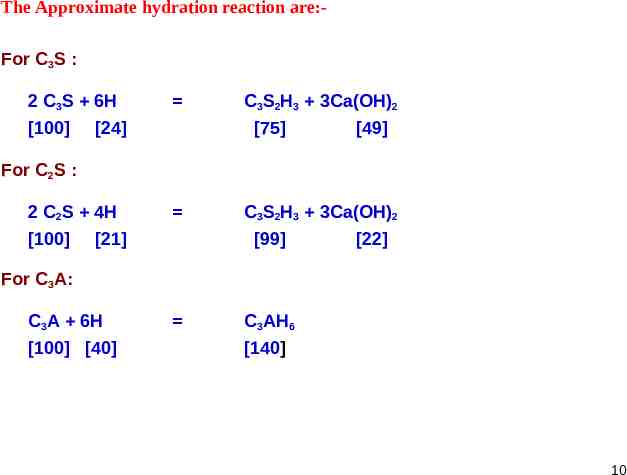
The Approximate hydration reaction are:For C3S : 2 C3S 6H [100] [24] C3S2H3 3Ca(OH)2 [75] [49] For C2S : 2 C2S 4H [100] [21] C3S2H3 3Ca(OH)2 [99] [22] For C3A: C3A 6H [100] [40] C3AH6 [140] 10
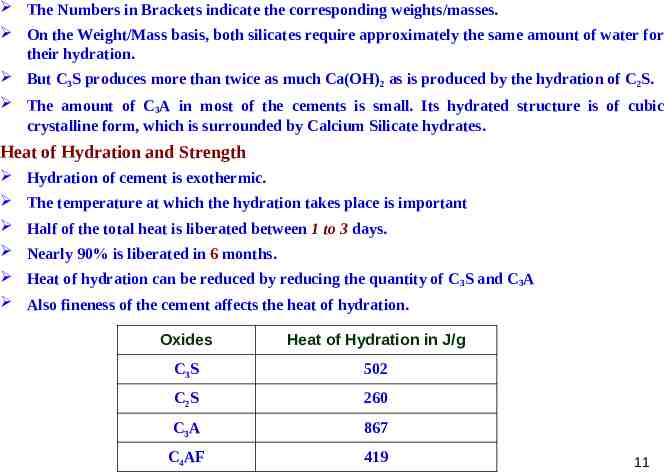
The Numbers in Brackets indicate the corresponding weights/masses. On the Weight/Mass basis, both silicates require approximately the same amount of water for their hydration. But C3S produces more than twice as much Ca(OH)2 as is produced by the hydration of C2S. The amount of C3A in most of the cements is small. Its hydrated structure is of cubic crystalline form, which is surrounded by Calcium Silicate hydrates. Heat of Hydration and Strength Hydration of cement is exothermic. The temperature at which the hydration takes place is important Half of the total heat is liberated between 1 to 3 days. Nearly 90% is liberated in 6 months. Heat of hydration can be reduced by reducing the quantity of C 3S and C3A Also fineness of the cement affects the heat of hydration. Oxides Heat of Hydration in J/g C3S 502 C2S 260 C3A 867 C4AF 419 11
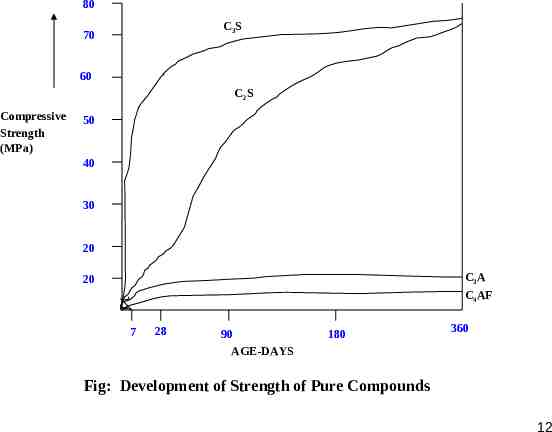
80 C3S 70 60 C2S Compressive Strength (MPa) 50 40 30 20 C3A 20 C4AF 7 28 90 AGE-DAYS 180 360 Fig: Development of Strength of Pure Compounds 12

C3S and C2S, which together constitute 70%-80% of cement, contribute to most of the strength developing characteristics of cement. High percentage of C3S (Low C2S) results in high early gain of strength accompanied by higher heat of hydration. Low C3S and high C2S leads to a slower rate of gain of strength with lesser heat of hydration. Though the long term strength of cement with any combination of C3S and C2S remain nearly the same. C3S contribute to most of the strength during first four weeks, C2S influences the strength after four weeks. Thus, their individual contribution to strength in long term(1 year) is equal for equal individual weights But, Lower rate of gain of strength leads to denser gel formation with high ultimate strength. In certain cements their might be a retrogression of strength in high C3S cements and continued increase in strength in high C2S cement over a longer period of time. This may be explained by the fact that hydration of C2S produces more of C3S2H3 and less Ca(OH)2 than that produced by C3S. 13

SECONDARY HYDRATION:- It is seen that Ca(OH)2 is produced during the hydration of C3S and C2S. This Ca(OH)2 is the weakest link since it is prone to chemical attack ultimately leading to Distress Leaching(seepage) Corrosion Cracks Expansion Finally leading to disintegration of concrete. Lime in highly corrosive environment leaches out of Concrete & Plaster, rendering the structure Porous. Certain type of siliceous materials, which are made reactive in finely divided form, are able to combine with lime at ordinary temperatures, to produce a binder similar in composition to that resulting from the hydration of Portland Cement. Ca(OH)2 SiO2 C3S2H3 This Secondary hydration reaction, continue over years and make the Concrete & Cement Plaster Dense. 14

C3S(3CaOSio2 H2O 3CaO.2Sio2.3H2O Ca(OH)2 Ca(OH)2 Sio2 3CaO.2SiO2.3H2O 15

Different Grades of Cement Higher grade of cements (higher than 33 grade) are produced by finer grinding and by increasing Tricalcium Silicate (C3S) content. Cements attaining high early strength are produced, by increasing the ratio of C3S/ C2S and the fineness. This leads high 28-day strength. The grade of cement refers to the compressive strength of mortar cube prepared with that cement & measured after 28 day of curing. The Compressive Strength of Cement of Grade 43 after 28 days of curing is expected to be 43 MPa. The long-term Strength of high and low-grade cements is the same. 16
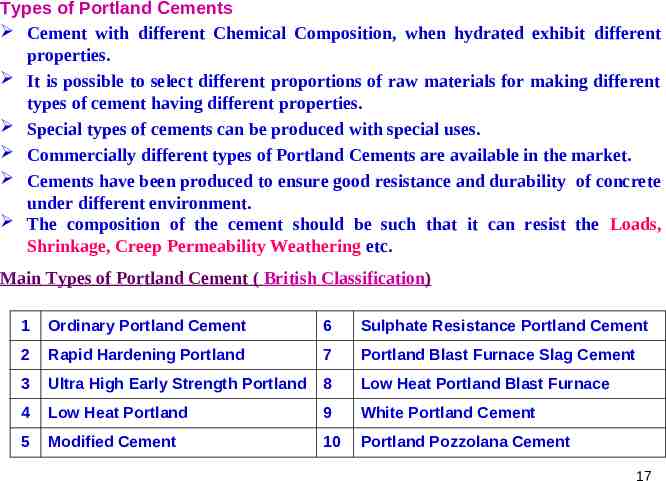
Types of Portland Cements Cement with different Chemical Composition, when hydrated exhibit different properties. It is possible to select different proportions of raw materials for making different types of cement having different properties. Special types of cements can be produced with special uses. Commercially different types of Portland Cements are available in the market. Cements have been produced to ensure good resistance and durability of concrete under different environment. The composition of the cement should be such that it can resist the Loads, Shrinkage, Creep Permeability Weathering etc. Main Types of Portland Cement ( British Classification) 1 Ordinary Portland Cement 6 Sulphate Resistance Portland Cement 2 Rapid Hardening Portland 7 Portland Blast Furnace Slag Cement 3 Ultra High Early Strength Portland 8 Low Heat Portland Blast Furnace 4 Low Heat Portland 9 White Portland Cement 5 Modified Cement 10 Portland Pozzolana Cement 17
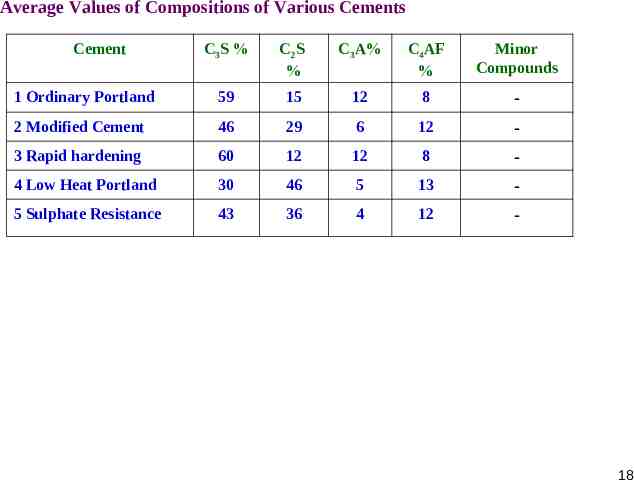
Average Values of Compositions of Various Cements Cement C3S % C2S % C3A% C4AF % Minor Compounds 1 Ordinary Portland 59 15 12 8 - 2 Modified Cement 46 29 6 12 - 3 Rapid hardening 60 12 12 8 - 4 Low Heat Portland 30 46 5 13 - 5 Sulphate Resistance 43 36 4 12 - 18






