Lab 4
12 Slides489.50 KB

Lab 4

Goals of the Experiment Measure bulk densities & calculate atomic densities of some transition metals Relate density to atomic size (a periodic trend)

Materials Cr (25 to 30 g) Mo (35 to 40 g) ZMo W (55 to 60 g) Forceps or tongs Water 10 ml graduated cylinder ZCr 24 42 ZW 74

Safety Gloves Safety goggles/glasses
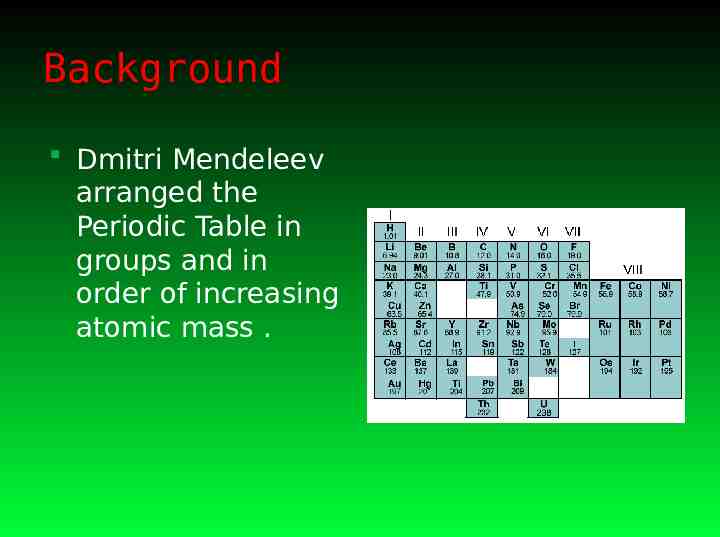
Background Dmitri Mendeleev arranged the Periodic Table in groups and in order of increasing atomic mass .

Background Henry Moseley rearranged the Periodic Table in order of increasing atomic number (Z).
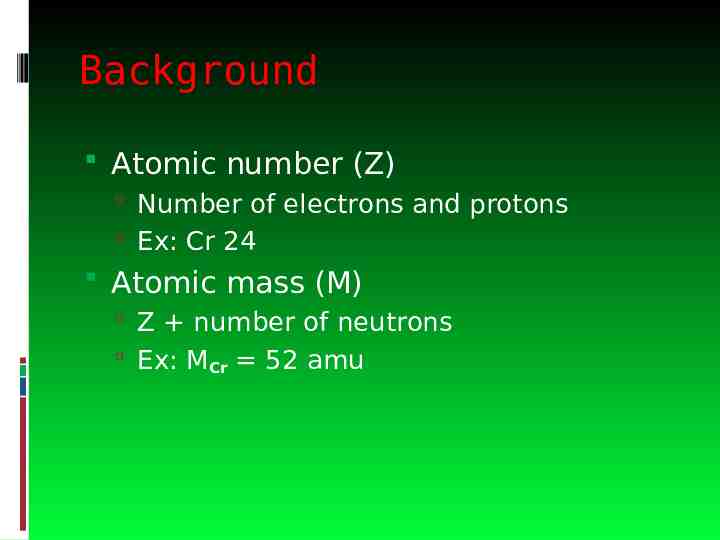
Background Atomic number (Z) Number of electrons and protons Ex: Cr 24 Atomic mass (M) Z number of neutrons Ex: MCr 52 amu

Background Atomic Mass (M) Some of the mass of an element is converted into energy (nuclear binding ), E mc2. Ex: Tungsten (Z 74; MW 184 amu). Actual mass (isotope) 183.95093129 amu. 1 amu 1.66 x 10-27 kg. (Show calculation)

Background (Periodic table) Property of an element depends on the location (family vertical column; period horizontal row).

Background (Periodic Trends) Atomic Radius Increases from R to L; Increases from Top to Bottom (Show schematic view) Ionization Energy (IE) – Emin required to remove 1 e- from an atom/ion in its ground state and it correlates to reactivity of metals (exceptions). Increases from L to R; Decreases as you go down a family Smaller IE more reactive the metal

Background (Periodic Trends) Electron affinity (EA) - E associated with the addition of an electron to an atom/ion & it correlates to the reactivity of nonmetals (exceptions). Increases from L to R; Decreases as you go down a family. Larger EA more reactive nonmetal

Background (Periodic Trends) Density also displays a periodic trend – atomic density increases from top to bottom but varies less significantly as one moves from left to right across a period. Bulk density depends on 3 properties: Mass of the atoms Packing arrangement (crystal structure – body centered, face centered, or simple cubic). Size of each atom






