Once-Daily Oral Semaglutide 50 mg Improves Cardiovascular Risk
17 Slides2.05 MB

Once-Daily Oral Semaglutide 50 mg Improves Cardiovascular Risk Factors in Overweight/Obesity in the OASIS 1 Trial W. Timothy Garvey,1 Thomas Holst-Hansen,2 Tobias Karlsson,2 Filip K. Knop,3 Naveen Rathor,2 Domenica Rubino,4 Julio Rosenstock5 Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; 2Novo Nordisk A/S, Søborg, Denmark; Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark; 4Washington Center for Weight Management and Research, Arlington, VA, USA; 5Velocity Clinical Research at Medical City, Dallas, TX, USA 1 3 This trial was sponsored by Novo Nordisk and is registered with ClinicalTrials.gov (NCT05035095). Medical writing assistance was provided by Casey McKeown RVN, FdSc, of Apollo, OPEN Health Communications, funded by Novo Nordisk A/S, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022). Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.

Disclosures W. Timothy Garvey: received grants from Novo Nordisk; served as site principal investigator for the clinical trial, which was sponsored by his university during the conduct of the study; receiving grants from Eli Lilly, Lexicon, and Pfizer outside the submitted work. He also served as a volunteer consultant on advisory committees for Boehringer Ingelheim, Jazz Pharmaceuticals, Novo Nordisk, Eli Lilly, and Pfizer. In each instance, he received no financial compensation, nor was there a financial relationship. Thomas Holst-Hansen, Tobias Karlsson, and Naveen Rathor: are employees of Novo Nordisk A/S. Filip K. Knop: received research grants, consultancy fees, and honoraria from, acted as an advisory board member for Novo Nordisk and Zealand Pharma; received consultancy fees and honoraria from, acted as an advisory board member for 89bio, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Structure Therapeutics, and Zucara; received consultancy fees and honoraria from Bayer and Pharmacosmos; and received research grants from Chr Hansen Domenica M. Rubino: received research grants, consultancy fees, travel fees, and honoraria from, acted as an advisory board member, speaker, and principal investigator for Novo Nordisk; has received research grants from, and is a principal investigator for Boehringer Ingelheim and Epitomee; and has received honoraria from the Endocrine Society, Medscape, and the PeerView Institute. Julio Rosenstock: received scientific advisory board fees, honoraria, consulting fees, and grants/research support from Novo Nordisk, Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Oramed, and Sanofi; honoraria or consulting fees from Zealand; received grants/research support from Genentech, Novartis, Pfizer, REMD Biotherapeutics, and vTv Therapeutics. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.

Background Pharmacotherapy for the treatment of overweight or obesity is a crucial aspect in the prevention and reduction of CVD risk1 The OASIS 1 trial investigated the efficacy and safety of oral semaglutide 50 mg taken once-daily plus lifestyle intervention in people with overweight or obesity 2 In OASIS 1, oral semaglutide 50 mg resulted in clinically-meaningful weight loss of 17.4% vs 1.8% (trial product estimand) with placebo with improvements in SBP, non-HDL cholesterol, and hsCRP in participants with overweight or obesity 2 This analysis aimed to evaluate the effect of oral semaglutide 50 mg vs placebo on CV risk factors according to weight loss category CV, cardiovascular; CVD, cardiovascular disease; HDL, high-density lipoprotein; hsCRP, high-sensitivity C‑reactive protein; OASIS, Oral Semaglutide Treatment Effect in People with Obesity; SBP, systolic blood pressure. 1. Arnett DK et al. Circulation 2019:140; e596–646; 2. Knop FK et al. The Lancet 2023:402(10403);705-719. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
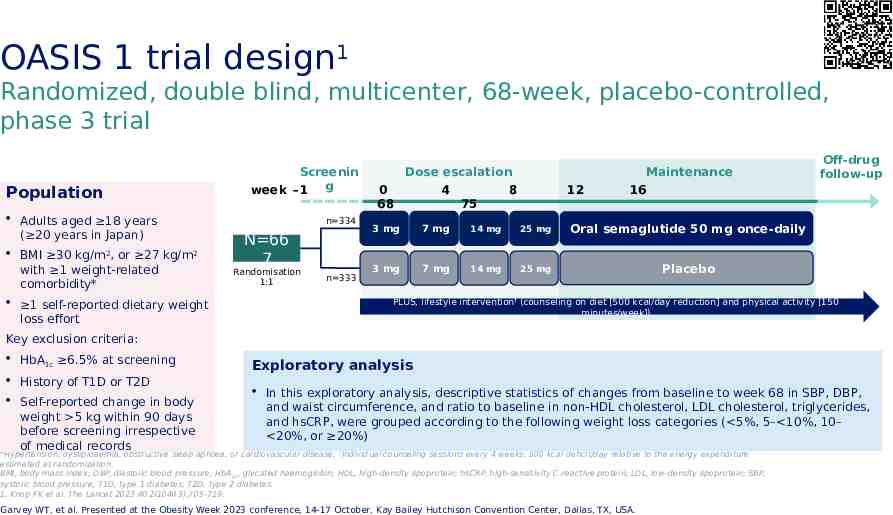
OASIS 1 trial design1 Randomized, double blind, multicenter, 68-week, placebo-controlled, phase 3 trial Population Screenin week –1 g Adults aged 18 years ( 20 years in Japan) BMI 30 kg/m2, or 27 kg/m2 with 1 weight-related comorbidity* 1 self-reported dietary weight loss effort n 334 N 66 7 Randomisation 1:1 n 333 Dose escalation 0 4 8 68 75 12 Maintenance 16 3 mg 7 mg 14 mg 25 mg Oral semaglutide 50 mg once-daily 3 mg 7 mg 14 mg 25 mg Placebo Off-drug follow-up PLUS, lifestyle intervention† (counseling on diet [500 kcal/day reduction] and physical activity [150 minutes/week]) Key exclusion criteria: HbA1c 6.5% at screening History of T1D or T2D Self-reported change in body weight 5 kg within 90 days before screening irrespective of medical records Exploratory analysis In this exploratory analysis, descriptive statistics of changes from baseline to week 68 in SBP, DBP, and waist circumference, and ratio to baseline in non-HDL cholesterol, LDL cholesterol, triglycerides, and hsCRP, were grouped according to the following weight loss categories ( 5%, 5– 10%, 10– 20%, or 20%) *Hypertension, dyslipidaemia, obstructive sleep apnoea, or cardiovascular disease; †Individual counseling sessions every 4 weeks; 500 kcal deficit/day relative to the energy expenditure estimated at randomization. BMI, body mass index; DBP, diastolic blood pressure; HbA 1c, glycated haemoglobin; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; SBP, systolic blood pressure; T1D, type 1 diabetes; T2D, type 2 diabetes. 1. Knop FK et al. The Lancet 2023:402(10403);705-719. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
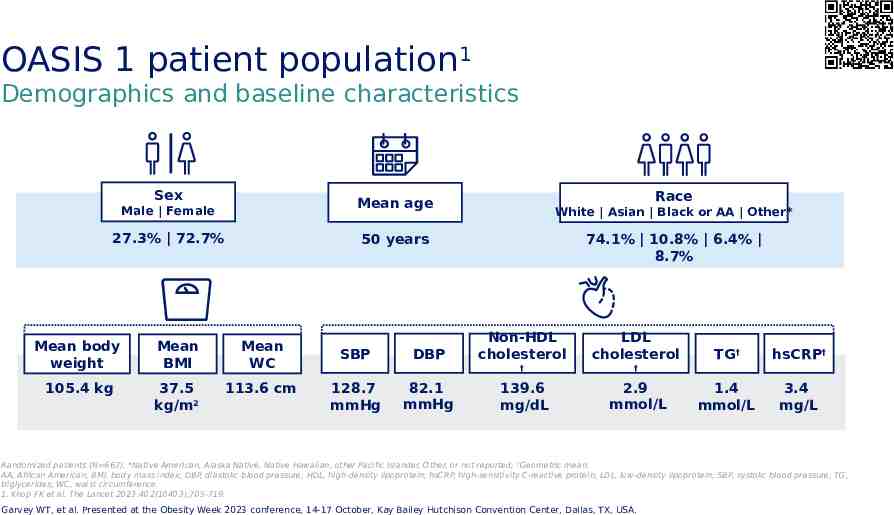
OASIS 1 patient population1 Demographics and baseline characteristics Sex Race Mean age Male Female 27.3% 72.7% White Asian Black or AA Other* 50 years Mean body weight Mean BMI Mean WC 105.4 kg 37.5 kg/m2 113.6 cm SBP 128.7 mmHg DBP 82.1 mmHg 74.1% 10.8% 6.4% 8.7% Non-HDL cholesterol LDL cholesterol † † 139.6 mg/dL 2.9 mmol/L TG† hsCRP† 1.4 mmol/L 3.4 mg/L Randomized patients (N 667). *Native American, Alaska Native, Native Hawaiian, other Pacific Islander, Other, or not reported; †Geometric mean. AA, African American; BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; SBP, systolic blood pressure; TG, triglycerides; WC, waist circumference. 1. Knop FK et al. The Lancet 2023:402(10403);705-719. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
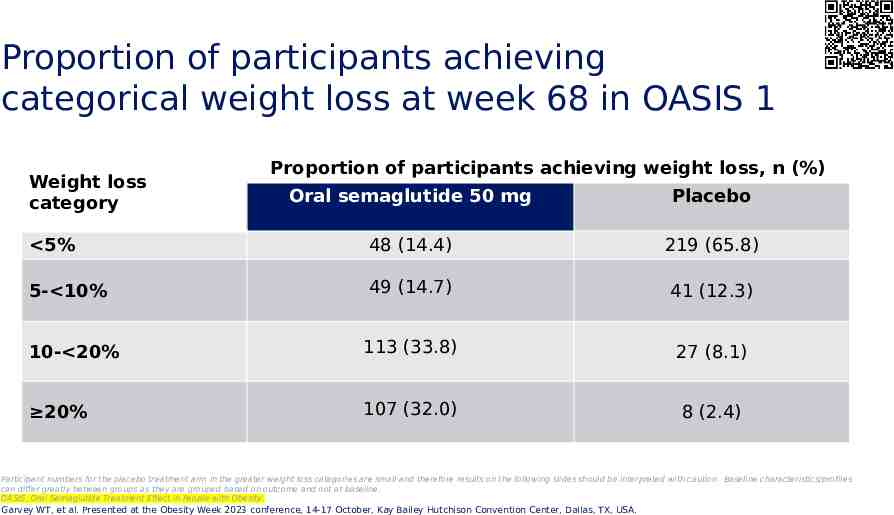
Proportion of participants achieving categorical weight loss at week 68 in OASIS 1 Weight loss category Proportion of participants achieving weight loss, n (%) Oral semaglutide 50 mg Placebo 5% 48 (14.4) 219 (65.8) 5- 10% 49 (14.7) 41 (12.3) 10- 20% 113 (33.8) 27 (8.1) 20% 107 (32.0) 8 (2.4) Participant numbers for the placebo treatment arm in the greater weight loss categories are small and therefore results on the following slides should be interpreted with caution. Baseline characteristics/profiles can differ greatly between groups as they are grouped based on outcome and not at baseline. OASIS, Oral Semaglutide Treatment Effect in People with Obesity. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
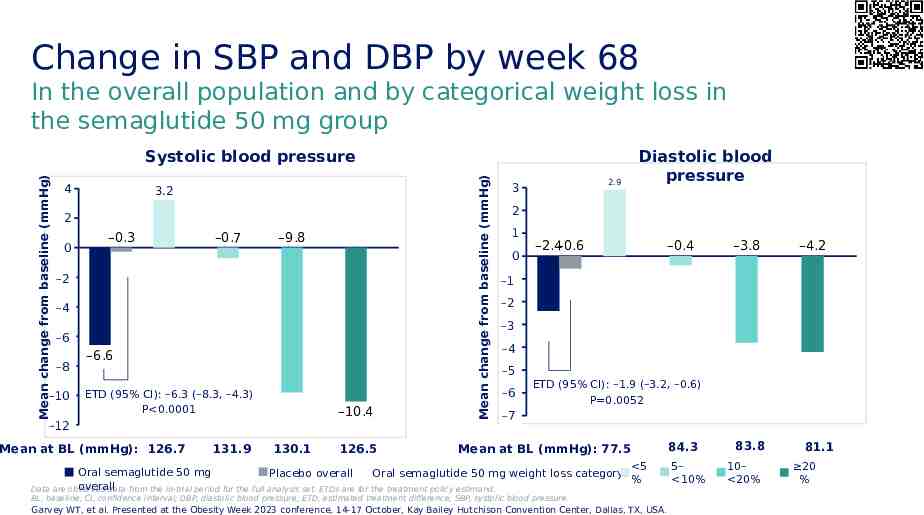
Change in SBP and DBP by week 68 In the overall population and by categorical weight loss in the semaglutide 50 mg group 4 3.2 2 0 –0.3 –0.7 –9.8 -2 –2 -4 –4 -6 –6 -8 –8 -10 –10 –6.6 ETD (95% CI): –6.3 (–8.3, –4.3) P 0.0001 –10.4 -12 –12 Mean at BL (mmHg): 126.7 131.9 130.1 126.5 Mean change from baseline (mmHg) Mean change from baseline (mmHg) Systolic blood pressure 2.9 3 Diastolic blood pressure 2 1 0 –2.4–0.6 –0.4 –3.8 –4.2 -1 –1 -2 –2 -3 –3 -4 –4 -5 –5 -6 –6 ETD (95% CI): –1.9 (–3.2, –0.6) P 0.0052 -7 –7 Mean at BL (mmHg): 77.5 5 Oral semaglutide 50 mg Oral semaglutide 50 mg weight loss category: Placebo overall % overall Data are observed data from the in-trial period for the full analysis set. ETDs are for the treatment policy estimand. BL, baseline; CI, confidence interval; DBP, diastolic blood pressure; ETD, estimated treatment difference; SBP, systolic blood pressure. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA. 84.3 5– 10% 83.8 10– 20% 81.1 20 %
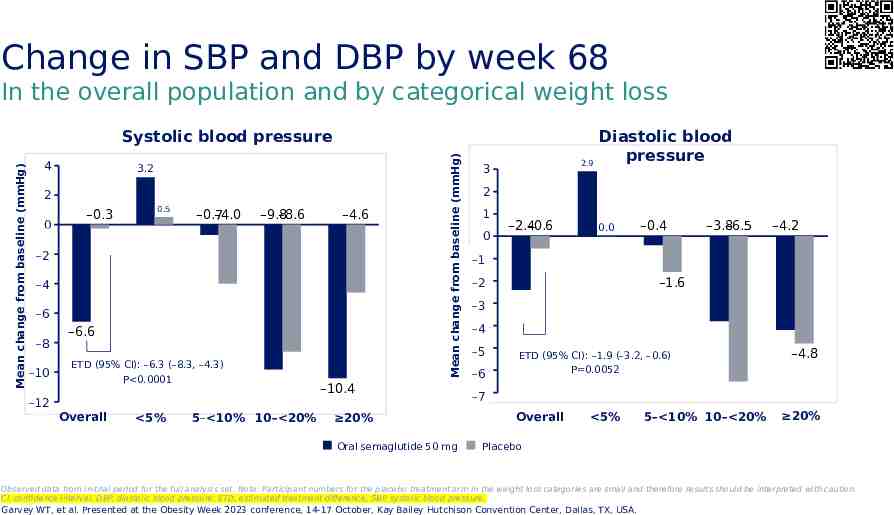
Change in SBP and DBP by week 68 In the overall population and by categorical weight loss 4 3.2 2 0 –0.3 0.5 –0.7 –4.0 –9.8 –8.6 –4.6 -2 –2 -4 –4 -6 –6 -8 –8 -10 –10 –6.6 ETD (95% CI): –6.3 (–8.3, –4.3) P 0.0001 -12 –12 Overall 5% 5– 10% 10– 20% Mean change from baseline (mmHg) Mean change from baseline (mmHg) Systolic blood pressure –10.4 20% Oral semaglutide 50 mg 2.9 3 Diastolic blood pressure 2 1 0 –2.4 –0.6 0.0 –0.4 –3.8 –6.5 –4.2 -1 –1 -2 –2 –1.6 -3 –3 -4 –4 -5 –5 -6 –6 ETD (95% CI): –1.9 (–3.2, –0.6) P 0.0052 –4.8 -7 –7 Overall 5% 5– 10% 10– 20% 20% Placebo Observed data from in-trial period for the full analysis set. Note: Participant numbers for the placebo treatment arm in the weight loss categories are small and therefore results should be interpreted with caution. CI, confidence interval; DBP, diastolic blood pressure; ETD, estimated treatment difference; SBP, systolic blood pressure. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
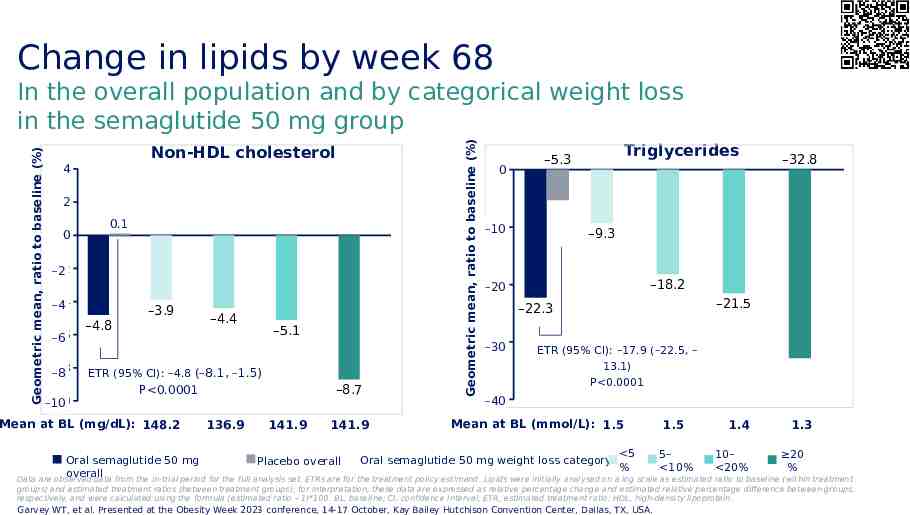
Change in lipids by week 68 Non-HDL cholesterol 4 2 0 0.1 -2 –2 -4 –4 -6 –6 -8 –8 -10 –10 –4.8 –3.9 –4.4 –5.1 ETR (95% CI): –4.8 (–8.1, –1.5) –8.7 P 0.0001 Mean at BL (mg/dL): 148.2 136.9 141.9 141.9 Geometric mean, ratio to baseline (%) Geometric mean, ratio to baseline (%) In the overall population and by categorical weight loss in the semaglutide 50 mg group 0 Triglycerides –5.3 -10 –10 –9.3 –18.2 -20 –20 –21.5 –22.3 -30 –30 –32.8 ETR (95% CI): –17.9 (–22.5, – 13.1) P 0.0001 -40 –40 Mean at BL (mmol/L): 1.5 1.5 1.4 1.3 5 5– 10– 20 Oral semaglutide 50 mg Oral semaglutide 50 mg weight loss category: Placebo overall % 10% 20% % overall Data are observed data from the in-trial period for the full analysis set. ETRs are for the treatment policy estimand. Lipids were initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula (estimated ratio – 1)*100. BL, baseline; CI, confidence interval; ETR, estimated treatment ratio; HDL, high-density lipoprotein. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
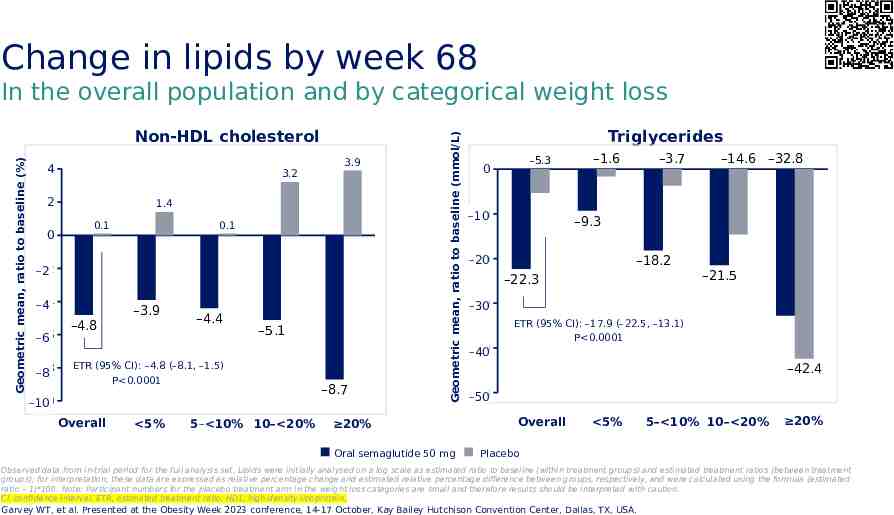
Change in lipids by week 68 Geometric mean, ratio to baseline (%) Non-HDL cholesterol 4 3.2 2 0 3.9 1.4 0.1 0.1 -2 –2 -4 –4 -6 –6 -8 –8 –4.8 –3.9 –4.4 –5.1 ETR (95% CI): –4.8 (–8.1, –1.5) P 0.0001 -10 –10 Overall 5% 5– 10% 10– 20% –8.7 Geometric mean, ratio to baseline (mmol/L) In the overall population and by categorical weight loss 20% Oral semaglutide 50 mg Triglycerides –5.3 0 -10 –10 –1.6 –3.7 –14.6 –32.8 –9.3 -20 –20 –18.2 –22.3 –21.5 -30 –30 -40 –40 ETR (95% CI): –17.9 (–22.5, –13.1) P 0.0001 –42.4 -50 –50 Overall 5% 5– 10% 10– 20% 20% Placebo Observed data from in-trial period for the full analysis set. Lipids were initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula (estimated ratio – 1)*100. Note: Participant numbers for the placebo treatment arm in the weight loss categories are small and therefore results should be interpreted with caution. CI, confidence interval; ETR, estimated treatment ratio; HDL, high-density lipoprotein. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
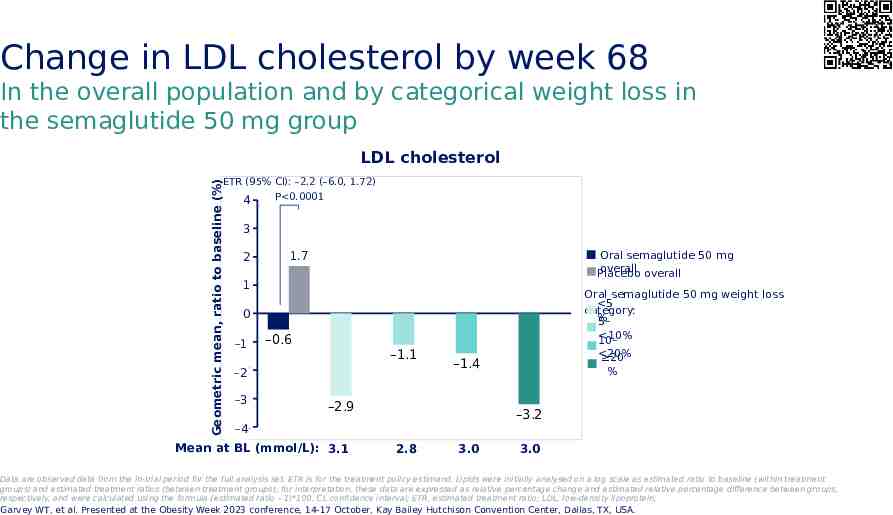
Change in LDL cholesterol by week 68 In the overall population and by categorical weight loss in the semaglutide 50 mg group LDL cholesterol Geometric mean, ratio to baseline (%) ETR (95% CI): –2.2 (–6.0, 1.72) P 0.0001 4 3 2 Oral semaglutide 50 mg overall overall Placebo 1.7 1 Oral semaglutide 50 mg weight loss 5 category: % 5– 10% 10– 0 -1 –1 –0.6 –1.1 -2 –2 -3 –3 –2.9 –3.2 -4 –4 Mean at BL (mmol/L): 3.1 20% 20 % –1.4 2.8 3.0 3.0 Data are observed data from the in-trial period for the full analysis set. ETR is for the treatment policy estimand. Lipids were initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula (estimated ratio – 1)*100. CI, confidence interval; ETR, estimated treatment ratio; LDL, low-density lipoprotein; Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
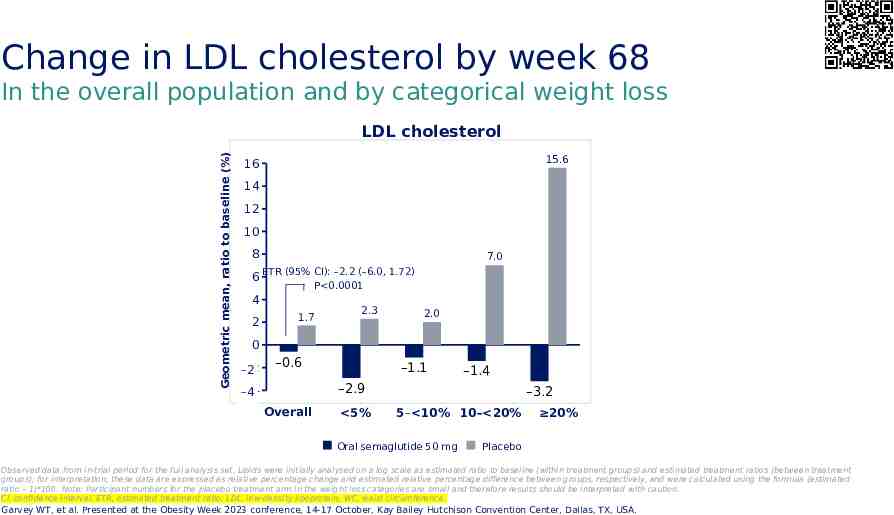
Change in LDL cholesterol by week 68 In the overall population and by categorical weight loss Geometric mean, ratio to baseline (%) LDL cholesterol 15.6 16 14 12 10 8 7.0 6 ETR (95% CI): –2.2 (–6.0, 1.72) P 0.0001 4 2 1.7 2.3 2.0 0 -2 –2 –0.6 –1.1 –1.4 –2.9 -4 –4 Overall 5% –3.2 5– 10% 10– 20% Oral semaglutide 50 mg 20% Placebo Observed data from in-trial period for the full analysis set. Lipids were initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula (estimated ratio – 1)*100. Note: Participant numbers for the placebo treatment arm in the weight loss categories are small and therefore results should be interpreted with caution. CI, confidence interval; ETR, estimated treatment ratio; LDL, low-density lipoprotein; WC, waist circumference. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
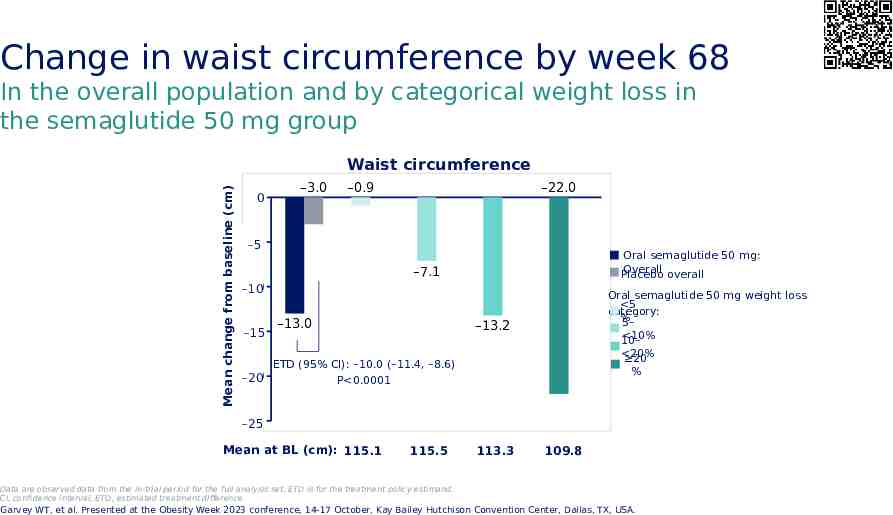
Change in waist circumference by week 68 In the overall population and by categorical weight loss in the semaglutide 50 mg group Mean change from baseline (cm) Waist circumference 0 –3.0 –0.9 –22.0 -5 –5 Oral semaglutide 50 mg: Overall overall Placebo –7.1 -10 –10 -15 –15 –13.0 Oral semaglutide 50 mg weight loss 5 category: % 5– 10% 10– –13.2 20% 20 % ETD (95% CI): –10.0 (–11.4, –8.6) -20 –20 P 0.0001 -25 –25 Mean at BL (cm): 115.1 115.5 113.3 109.8 Data are observed data from the in-trial period for the full analysis set. ETD is for the treatment policy estimand. CI, confidence interval; ETD, estimated treatment difference Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
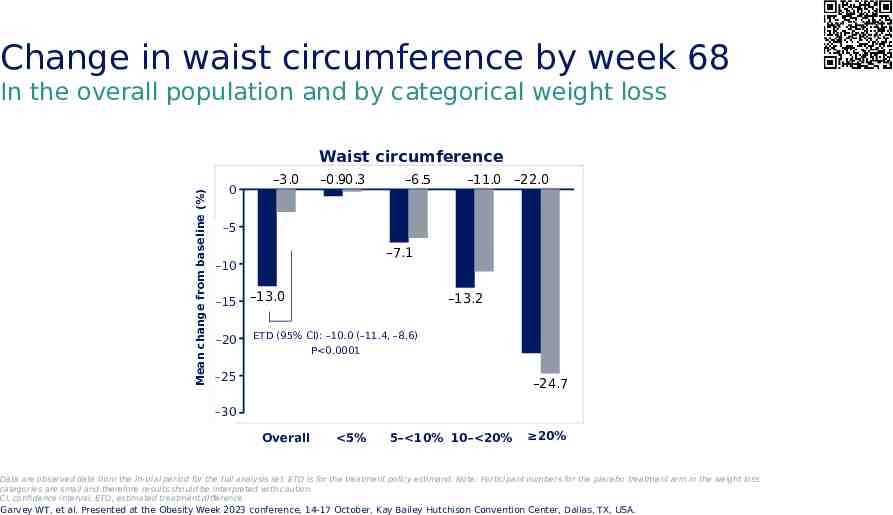
Change in waist circumference by week 68 In the overall population and by categorical weight loss Mean change from baseline (%) Waist circumference 0 –3.0 –0.9 –0.3 –6.5 –11.0 –22.0 -5 –5 –7.1 -10 –10 -15 –15 -20 –20 –13.0 –13.2 ETD (95% CI): –10.0 (–11.4, –8.6) P 0.0001 -25 –25 –24.7 –30 -30 Overall 5% 5– 10% 10– 20% 20% Data are observed data from the in-trial period for the full analysis set. ETD is for the treatment policy estimand. Note: Participant numbers for the placebo treatment arm in the weight loss categories are small and therefore results should be interpreted with caution. CI, confidence interval; ETD, estimated treatment difference Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
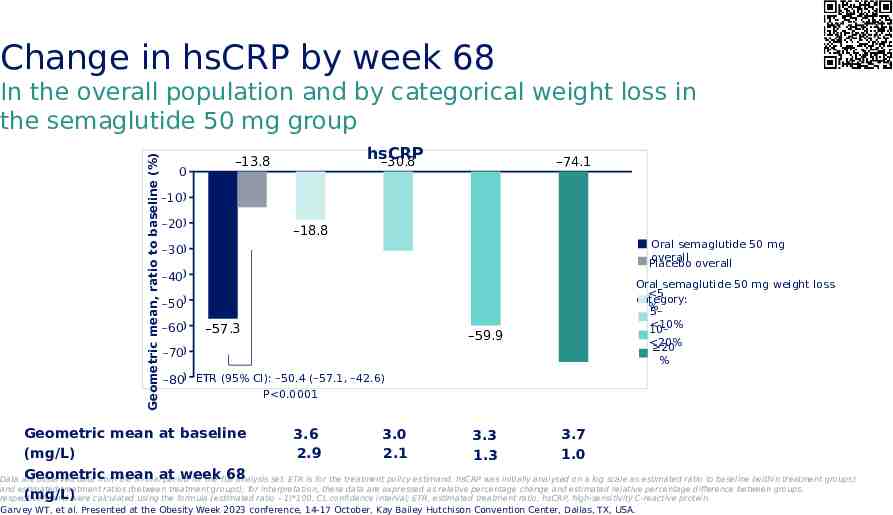
Change in hsCRP by week 68 Geometric mean, ratio to baseline (%) In the overall population and by categorical weight loss in the semaglutide 50 mg group 0 hsCRP –30.8 –13.8 –74.1 -10 –10 -20 –20 –18.8 Oral semaglutide 50 mg overall overall Placebo -30 –30 -40 –40 -50 –50 -60 –60 –57.3 –59.9 Oral semaglutide 50 mg weight loss 5 category: % 5– 10% 10– -70 –70 20% 20 % -80 ETR (95% CI): –50.4 (–57.1, –42.6) –80 P 0.0001 Geometric mean at baseline 3.0 3.6 3.7 3.3 2.1 2.9 (mg/L) 1.0 1.3 Geometric mean week 68 Data are observed data from the in-trialat period for the full analysis set. ETR is for the treatment policy estimand. hsCRP was initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, (mg/L) respectively, and were calculated using the formula (estimated ratio – 1)*100. CI, confidence interval; ETR, estimated treatment ratio; hsCRP, high-sensitivity C-reactive protein. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.
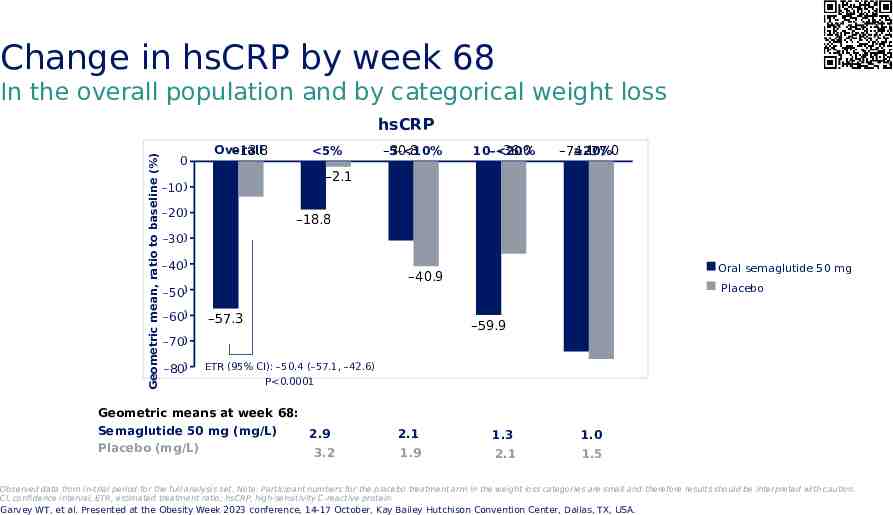
Change in hsCRP by week 68 In the overall population and by categorical weight loss Geometric mean, ratio to baseline (%) hsCRP 0 Overall –13.8 5% 5– 10% –30.8 10– 20% –36.0 20% –74.1 –77.0 –2.1 -10 –10 -20 –20 –18.8 -30 –30 -40 –40 -50 –50 -60 –60 Oral semaglutide 50 mg –40.9 –57.3 Placebo –59.9 -70 –70 -80 –80 ETR (95% CI): –50.4 (–57.1, –42.6) P 0.0001 Geometric means at week 68: Semaglutide 50 mg (mg/L) 2.9 Placebo (mg/L) 3.2 2.1 1.3 1.0 1.9 2.1 1.5 Observed data from in-trial period for the full analysis set. Note: Participant numbers for the placebo treatment arm in the weight loss categories are small and therefore results should be interpreted with caution. CI, confidence interval; ETR, estimated treatment ratio; hsCRP, high-sensitivity C-reactive protein. Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.

Conclusions Once-daily oral semaglutide 50 mg was associated with meaningful reductions in CV risk factors from baseline to week 68 The greatest improvements in CV risk factors occurred in participants with the greatest weight loss for semaglutide 50 mg Improvements in blood pressures, triglycerides, non-HDL cholesterol and hsCRP were progressive with increasing weight loss, up to and including 20% CV, cardiovascular Garvey WT, et al. Presented at the Obesity Week 2023 conference, 14-17 October, Kay Bailey Hutchison Convention Center, Dallas, TX, USA.






