Blood Transfusion – Safety, optimisation & new advances Dr Shubha
24 Slides8.65 MB

Blood Transfusion - Safety, optimisation & new advances Dr Shubha Allard Consultant Haematologist Barts Health NHS Trust and NHS Blood and Transplant

NHS Blood and Transplant NHS Blood and Transplant (NHSBT) manages the national voluntary donation system for blood, tissues, organs and stem cells Supplies around 2 million units of blood a year

Blood safety/ Transfusion safety SAFE TRANSFUSION PROCESS SAFE BLOOD COMPONENT

Blood Transfusion -Guidance and Regulations WHO recommendations safe and adequate blood supply also clinical transfusion process – – – – – – Appropriate use of blood Collection samples, patient ID compatibility testing Administration of blood Adverse event reporting Hospital transfusion committee ‘Better Blood Transfusion’ EU Optimal Blood Use manual (www.optimalblooduse.eu) Council of Europe 47 member countries

European Union Blood Directives Setting standards of quality and safety for collection, testing, processing, storage and distribution of human blood and blood components Blood Safety and Quality Regulations 2005 – Transposed into UK law Regulations affect the blood services (called blood establishments) and hospital transfusion laboratories (hospital blood banks) Competent Authority – Medicines and Healthcare products Regulatory Agency (MHRA)

Blood safety and Quality Regulations impact on hospitals in the UK Quality management system Stringent requirements storage/distribution blood -‘cold chain’ standard operating procedures (SOPs) Corrective and preventative action (CAPA) Validation & change control – Traceability – Training and competency assessment – Haemovigilance annual statement of compliance to MHRA 60 hospitals inspected per year – ‘Cease & desist’ – Critical non -compliances

Safety of the Blood Supply Voluntary and non-remunerated donor Donor Health Questionnaire Council of Europe - Mandatory screening tests – Hep B, Hep C, HIV 1 & 2 – Additional testing – Syphilis, HTLV – Selective screening – Malaria, CMV
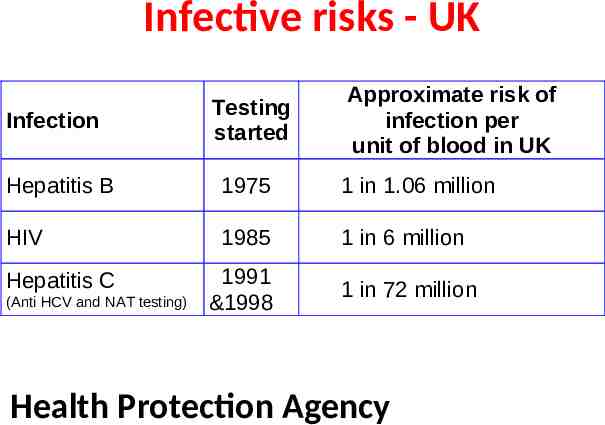
Infective risks - UK Infection Testing started Approximate risk of infection per unit of blood in UK Hepatitis B 1975 1 in 1.06 million HIV 1985 1 in 6 million 1991 &1998 1 in 72 million Hepatitis C (Anti HCV and NAT testing) Health Protection Agency
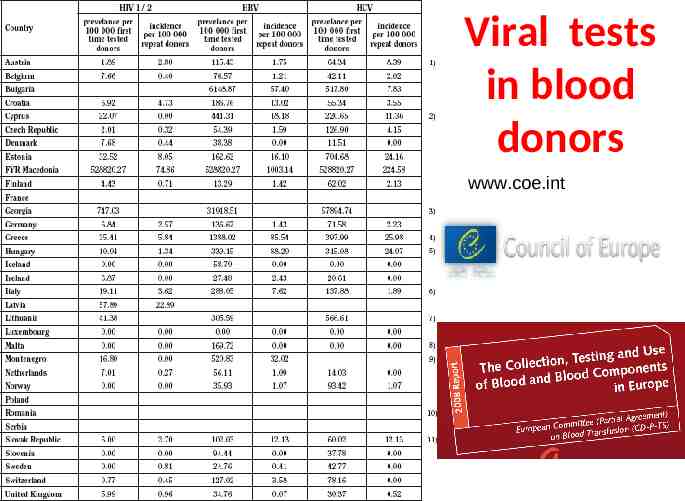
Viral tests in blood donors www.coe.int
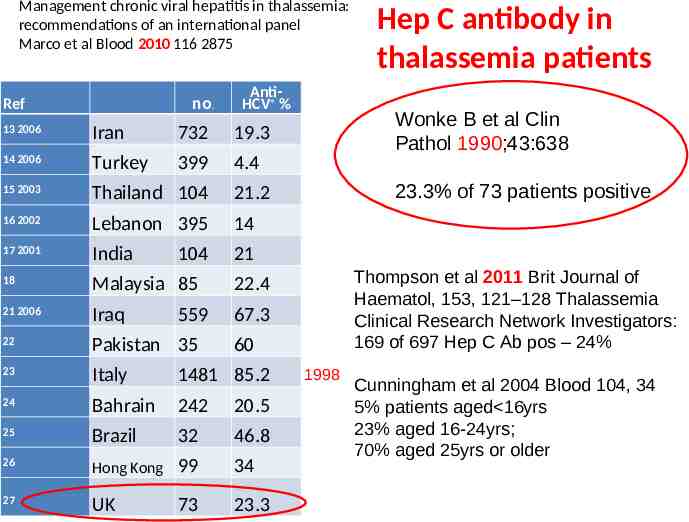
Management chronic viral hepatitis in thalassemia: recommendations of an international panel Marco et al Blood 2010 116 2875 no. Ref AntiHCV % 13 2006 Iran 732 19.3 14 2006 Turkey 399 4.4 15 2003 Thailand 104 21.2 16 2002 Lebanon 395 14 17 2001 India 21 18 Malaysia 85 22.4 21 2006 Iraq 67.3 22 Pakistan 35 23 Italy 1481 85.2 24 Bahrain 242 20.5 25 Brazil 32 46.8 26 Hong Kong 99 34 27 UK 73 23.3 104 559 Hep C antibody in thalassemia patients Wonke B et al Clin Pathol 1990;43:638 23.3% of 73 patients positive Thompson et al 2011 Brit Journal of Haematol, 153, 121–128 Thalassemia Clinical Research Network Investigators: 169 of 697 Hep C Ab pos – 24% 60 1998 Cunningham et al 2004 Blood 104, 34 5% patients aged 16yrs 23% aged 16-24yrs; 70% aged 25yrs or older
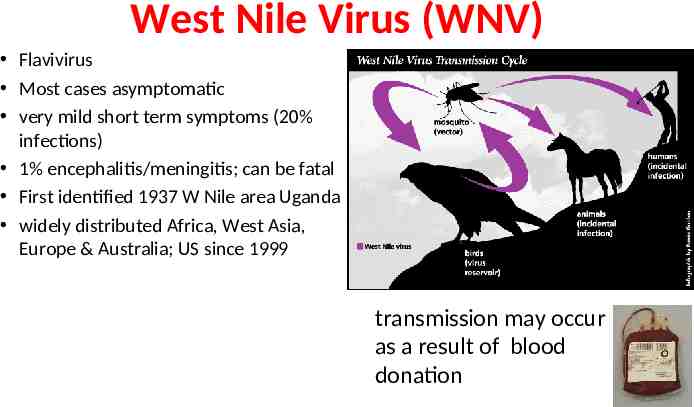
West Nile Virus (WNV) Flavivirus Most cases asymptomatic very mild short term symptoms (20% infections) 1% encephalitis/meningitis; can be fatal First identified 1937 W Nile area Uganda widely distributed Africa, West Asia, Europe & Australia; US since 1999 transmission may occur as a result of blood donation

WNV – blood donation EU Directive - deferral for 28 days - No provision for WNV Nucleic acid testing (NAT) in place of deferral Since 2005 UK blood services have deferred travellers – Concerns re impact on blood supply – planning 2012 Olympic s – MHRA accepted WNV NAT testing rather than donor deferral – May- Aug 2012 NHSBT has tested 13,000 donations - so far all negative West Nile Virus and Blood Safety Introduction to a Preparedness Plan in Europe – EU satellite meeting Working Group on Blood Safety; Jan 2011 – Surveillance, Risk assessment, Deferral criteria, NAT testing, Impact on blood supply
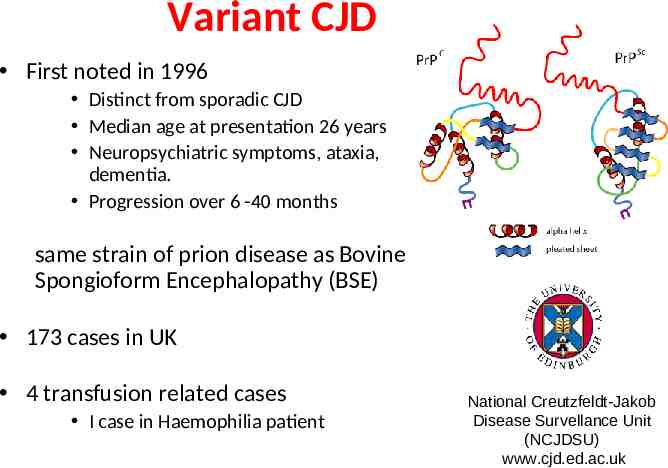
Variant CJD First noted in 1996 Distinct from sporadic CJD Median age at presentation 26 years Neuropsychiatric symptoms, ataxia, dementia. Progression over 6 -40 months same strain of prion disease as Bovine Spongioform Encephalopathy (BSE) 173 cases in UK 4 transfusion related cases I case in Haemophilia patient National Creutzfeldt-Jakob Disease Survellance Unit (NCJDSU) www.cjd.ed.ac.uk
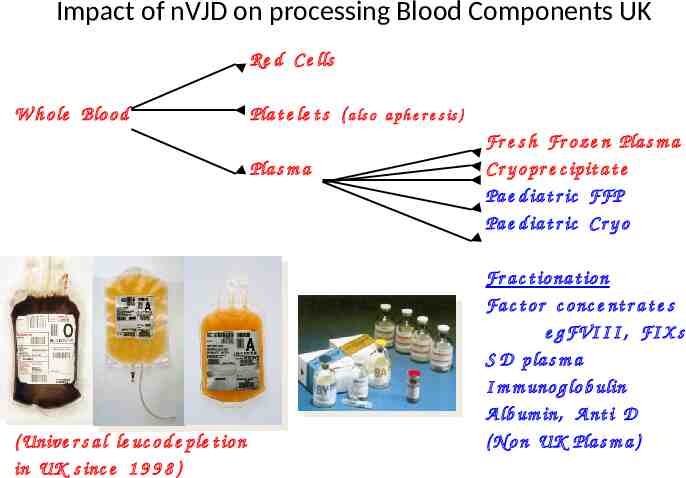
Impact of nVJD on processing Blood Components UK Re d C e lls W h o le Blo o d Pla t e le t s ( a ls o a ph e r e s is ) Pla s m a ( Unive r s a l le uc o d e ple t io n in UK s inc e 1 9 9 8 ) Fr e s h Fr o z e n Pla s m a C r y o pr e c ipit a t e Pa e d ia t r ic FFP Pa e d ia t r ic C r y o Fr a c t io na t io n Fa c t o r c o nc e nt r a t e s e g FVI I I , FI X s S D p la s m a I m m uno g lo b ulin A lb um in, A nt i D ( N o n UK Pla s m a )

Blood processing – red cells Most of the plasma is removed Optimal Additive Solution added SAGM in UK Red Book Specifications Vol 280 60ml WBC 5 x 106 /unit Hct 0.5 - 0.7 35 day shelf life For haemoglobinopathy Top up 14 days exchange (SCD) 7 days (washed red cells)

Serious Hazards of Transfusion (SHOT) UK-wide, established1996 confidential reporting evidence base to support – blood safety policy decisions – clinical guidelines & education – improvements in transfusion practice
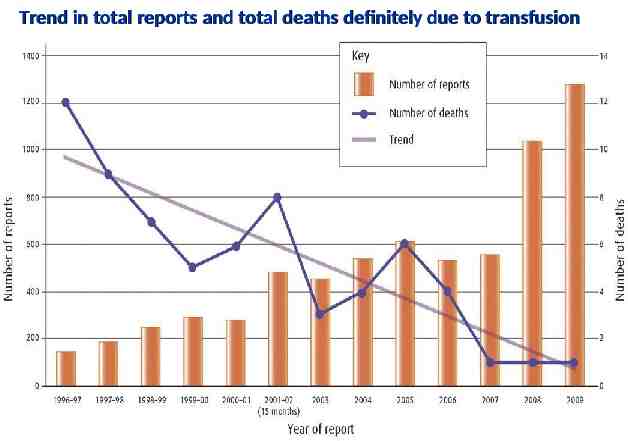
Trend in total reports and total deaths definitely due to transfusion
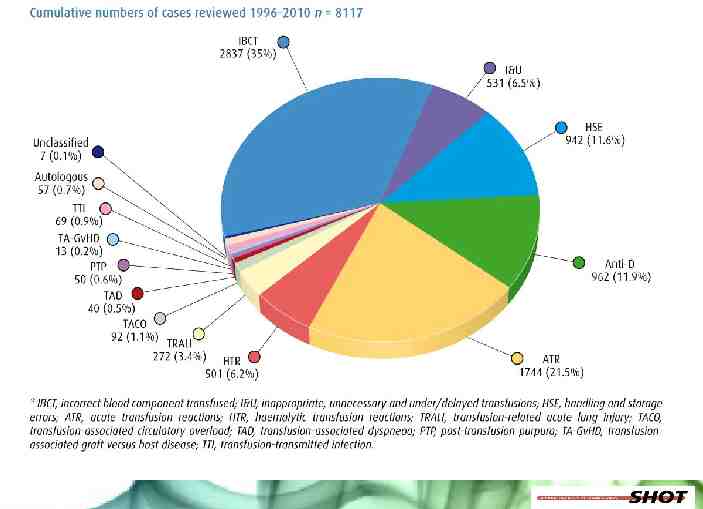
Special requirements Haemoglobinopathy TIF Guidelines, UK Standards thalassaemia, Sickle Cell Disease British Committee Standards Haematology (www.bcsh.org) Red cells matched for Rh (D, C, c, E, e) and K antigens Antigen negative for current or historical red cell antibodies that are clinically significant patient’s red cells phenotyped prior to transfusion or molecular genotyping if transfused – C, c, E, e, K, k, Jka, Jkb, Fya, Fyb, MNS

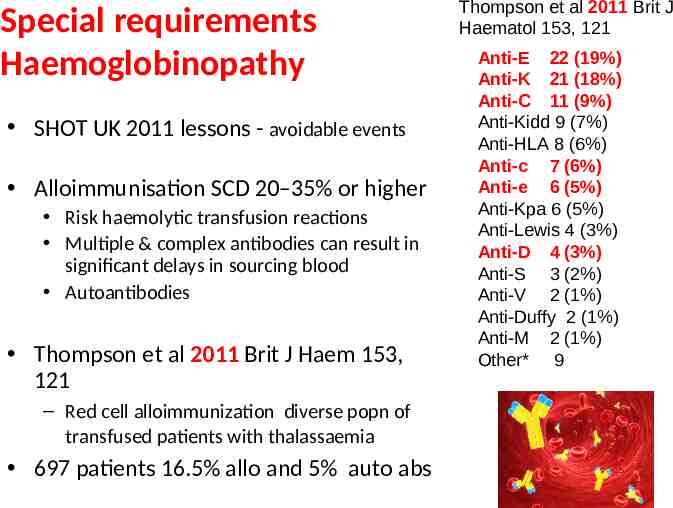
Special requirements Haemoglobinopathy SHOT UK 2011 lessons - avoidable events Alloimmunisation SCD 20–35% or higher Risk haemolytic transfusion reactions Multiple & complex antibodies can result in significant delays in sourcing blood Autoantibodies Thompson et al 2011 Brit J Haem 153, 121 – Red cell alloimmunization diverse popn of transfused patients with thalassaemia 697 patients 16.5% allo and 5% auto abs Thompson et al 2011 Brit J Haematol 153, 121 Anti-E 22 (19%) Anti-K 21 (18%) Anti-C 11 (9%) Anti-Kidd 9 (7%) Anti-HLA 8 (6%) Anti-c 7 (6%) Anti-e 6 (5%) Anti-Kpa 6 (5%) Anti-Lewis 4 (3%) Anti-D 4 (3%) Anti-S 3 (2%) Anti-V 2 (1%) Anti-Duffy 2 (1%) Anti-M 2 (1%) Other* 9
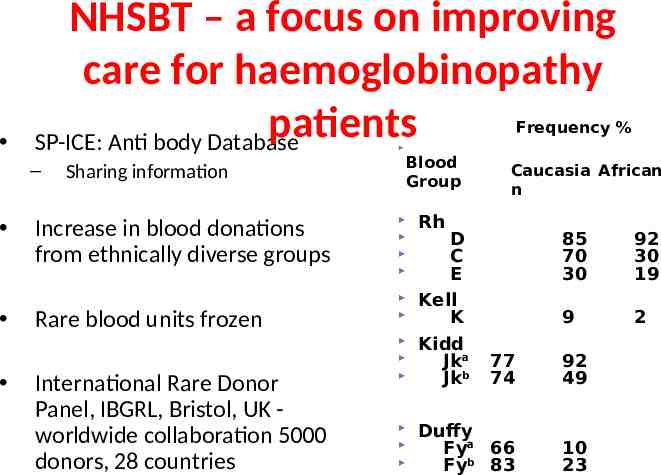
NHSBT – a focus on improving care for haemoglobinopathy patients SP-ICE: Anti body Database Frequency % – Sharing information Increase in blood donations from ethnically diverse groups Rare blood units frozen International Rare Donor Panel, IBGRL, Bristol, UK worldwide collaboration 5000 donors, 28 countries Blood Caucasia African frequency (%Blood group Group CaucasAfricanian African n Rh D C E Kell K Kidd Jka Jkb 85 70 30 92 30 19 9 2 77 74 92 49 Duffy Fya 66 Fyb 83 10 23
Molecular techniques Extended red cell matching Automated, high throughput testing platforms – now available for molecular testing ?scope for extended donor testing and greater red cell antigen matching with recipient NHS Blood and Transplant - Evaluation of chip based and Luminex based genotyping platforms – Panel of 1,000 DNA samples from donors with known phenotype – Inter platform discrepancy very low (0.04%) across 20,000 blood groupings – Pilot H&I and RCI labs implementation patient testing
Red cells from stem cells Harvey G. Klein. Brewing blood Blood 2011 118, 5069 Standardised well characterised, readily available red cells? Culture systems to generate erythroid cells in laboratory from Somatic stem cells Embryonic stem cells Induced pluripotent stem cells Ex vivo production of human red cells, the Holy Grail of blood transfusion. illustration by Debra T. Dartez.
Blood safety, optimisation and new advances Transfusion transmitted infections – Reduced rates, never zero risk, emerging infections Adherence to guidelines – avoidable alloimunisation Haemovigilance – Essential for improving transfusion safety – Highlights areas for action New advances – e.g. IT, molecular techniques, pathogen activation







