Antimicrobial Resistance: Developing and testing innovative models for
24 Slides453.45 KB

Antimicrobial Resistance: Developing and testing innovative models for the evaluation and purchase of antimicrobials. In support of the UK’s vision for AMR by 2040 and five-year national action plan January2021

Housekeeping Duration of webinar is approximately two hours Please note that this webinar, including Q&A will be recorded and may be published later. Questions should be submitted using the ‘chat’ function. We will look to collate these and provide answers at the end of each section. 2

Key updates Product selected for the new antimicrobial part of the project: Cefiderocol (Fetcroja) manufactured by Shionogi Product selected for the existing antimicrobial part of the project: Ceftazidime with avibactam (Zavicefta) manufactured by Pfizer 3

Project overview Aim: To develop and test an innovative payment model that reimburses companies for antimicrobials based primarily on their value to the NHS as opposed to volumes used. Success criteria: An agreed HTA valuation framework and complete value assessment of two products. An agreed payment framework that leads to successful negotiation of payments for the two products, supporting good stewardship. Other countries test models that, together, achieve pull incentives for antimicrobials and stimulate companies to increase investment. 4

Background In 2015, a joint Government/ Industry AMR working group and a sub-group were established to consider reimbursement and evaluation of antimicrobials and the principle of an innovative payment model was agreed. In 2017, The Economic Evaluation Policy Research Unit (EEPRU) was commissioned to consider a value assessment approach for antimicrobials. In October 2018, EEPRU published a “Framework for value assessment of new antimicrobials”. The UK five-year national action plan for AMR published in January 2019 commits to test a new evaluation and payment model for antimicrobials. In 2019 and early 2020, NHS England & Improvement and NICE in conjunction with feedback from industry and international stakeholders, finalised proposals to evaluate antimicrobials based upon their value to the NHS and to reimburse based upon value rather than the quantity supplied. In June 2020, a tender for one existing antimicrobial and one new antimicrobial was launched by NHS England and NHS Improvement / NICE project team In December 2020, the process of selecting two antimicrobials to proceed to the NICE evaluation process and ultimately to be supplied via the new payment model was completed. 5
The payment model The principle of the model is that companies are paid for antimicrobials based on the estimated value of benefits to patients and the NHS which is consistent with good stewardship rather than payments based on volumes used. The value of the antimicrobials will be estimated by NICE through an adapted Health Technology Assessment with information from health economic modelling and expert opinion. The model will capture value not just from the direct health gain to patients treated, but additional elements e.g. insurance value, diversity value, transmission value and enablement value Value assessment will be used to inform commercial discussions, leading to payments to companies of an annual fixed fee in instalments. 6

Application of the model The model is being tested through application to two antimicrobial products selected via competitive procurement exercise: One existing (has received marketing authorisation in past 2-3 years) One new to market (with expectation of a marketing authorisation by end 2020 and with plans to launch in UK) Future policy for the evaluation and purchasing of antimicrobials will be informed by the outcomes from this project 7
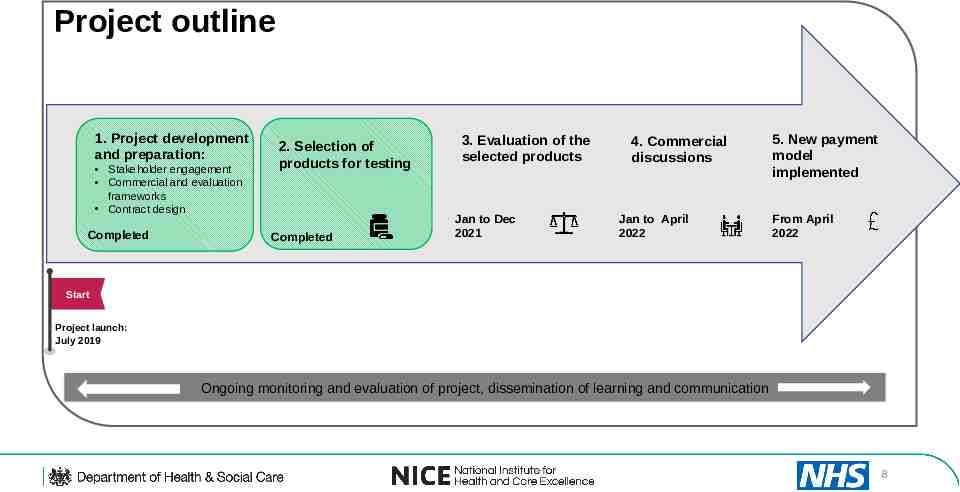
Project outline 1. Project development and preparation: Stakeholder engagement Commercial and evaluation frameworks Contract design Completed 2. Selection of products for testing Completed 3. Evaluation of the selected products Jan to Dec 2021 4. Commercial discussions Jan to April 2022 5. New payment model implemented From April 2022 Start Project launch: July 2019 Ongoing monitoring and evaluation of project, dissemination of learning and communication 8

The Selection Process 9
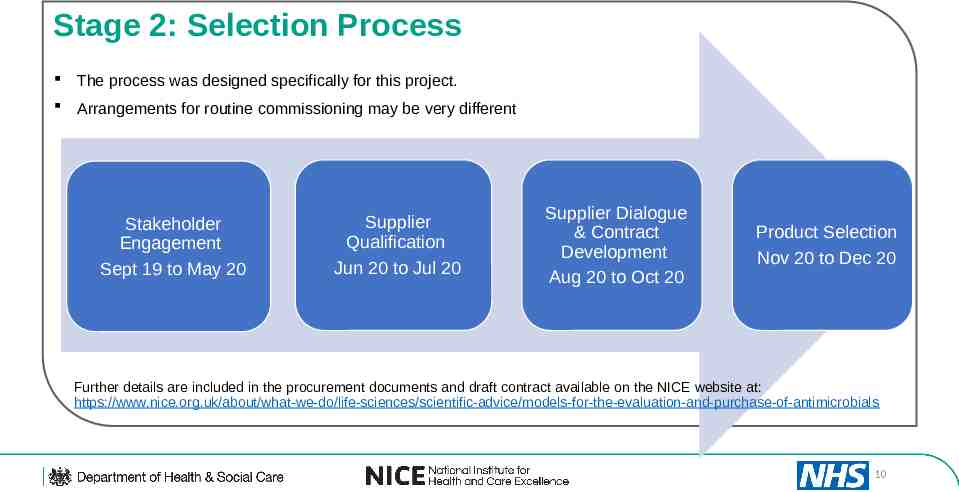
Stage 2: Selection Process The process was designed specifically for this project. Arrangements for routine commissioning may be very different Stakeholder Engagement Sept 19 to May 20 Supplier Qualification Jun 20 to Jul 20 Supplier Dialogue & Contract Development Aug 20 to Oct 20 Product Selection Nov 20 to Dec 20 Further details are included in the procurement documents and draft contract available on the NICE website at: https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials 10

Selection Process: Stakeholder Engagement Significant engagement with industry and international stakeholders that informed the process Payment model largely based upon industry proposals THANK YOU to all those who contributed 11
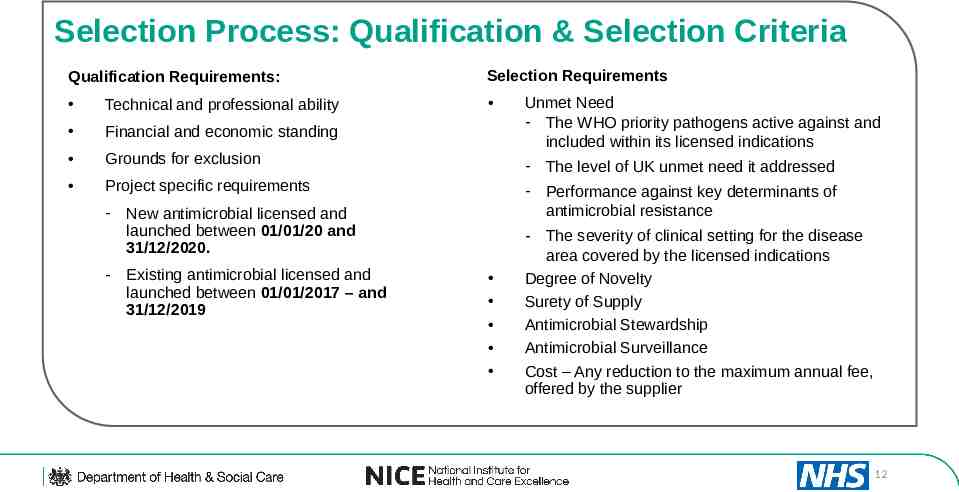
Selection Process: Qualification & Selection Criteria Qualification Requirements: Selection Requirements Technical and professional ability Financial and economic standing Grounds for exclusion Project specific requirements - The level of UK unmet need it addressed - Performance against key determinants of antimicrobial resistance - New antimicrobial licensed and launched between 01/01/20 and 31/12/2020. - Existing antimicrobial licensed and launched between 01/01/2017 – and 31/12/2019 Unmet Need - The WHO priority pathogens active against and included within its licensed indications - The severity of clinical setting for the disease area covered by the licensed indications Degree of Novelty Surety of Supply Antimicrobial Stewardship Antimicrobial Surveillance Cost – Any reduction to the maximum annual fee, offered by the supplier 12

Selection Process: Supplier Dialogue Individual supplier meetings were invaluable to: Develop the final form contract e.g. - Payment model - Surety of supply - Stewardship and surveillance requirements Ensure understanding and reach agreement between suppliers and ourselves e.g. - An antimicrobials anticipated usage - A price (subject to NICE evaluation) that hospitals would purchase the antimicrobial - Performance requirements 13

Selection Process: Outputs Two products selected An agreed subscription type payment model A contract ready for signing, subject to inserting the following that will be informed by the outcome of the NICE evaluation: The annual contract value The confidential hospital purchase price per pack Any product specific adjustments to the stewardship & surveillance requirements 14

Selection Process: – Contract Highlights Subscription type payment model (independent of quantity supplied) Contract value set based upon value to NHS subject to cap of 10m pa 3-10 year term Pressure relief mechanism in event of very significant and unanticipated increased usage Confidential hospital purchase price to facilitate use of existing supply chains Hospital purchase price to be set to encourage appropriate use and discourage inappropriate use and can be adjusted by mutual agreement. A limited number of performance requirements Minimum On Time in Full (OTIF) Minimum stock holding within supply chain and physically within England Compliance with stewardship, manufacturing, environmental and surveillance requirements 15

Evaluation of the selected products 16
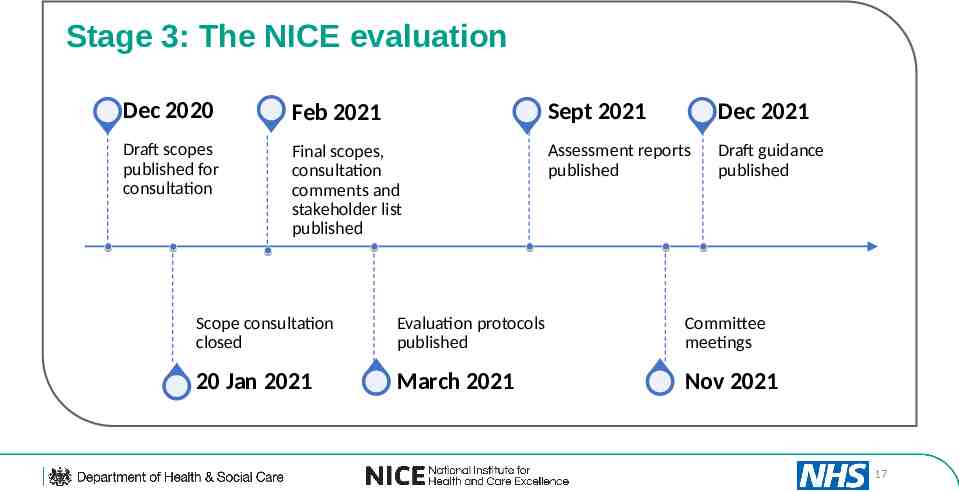
Stage 3: The NICE evaluation Dec 2020 Feb 2021 Sept 2021 Dec 2021 Draft scopes published for consultation Final scopes, consultation comments and stakeholder list published Assessment reports published Draft guidance published Scope consultation closed Evaluation protocols published Committee meetings 20 Jan 2021 March 2021 Nov 2021 17

Selected products for NICE evaluation Cefiderocol – marketing authorisation Infections due to aerobic Gram-negative organisms in adults with limited treatment options Ceftazidime with avibactam – marketing authorisation Complicated intra-abdominal infection Complicated urinary tract infection, including pyelonephritis Hospital-acquired pneumonia, including ventilator associated pneumonia Bacteraemia that occurs in association with, or is suspected to be associated with, any of the infections listed above Infections due to aerobic Gram-negative organisms in adults and paediatric patients aged 3 months and older with limited treatment options 18
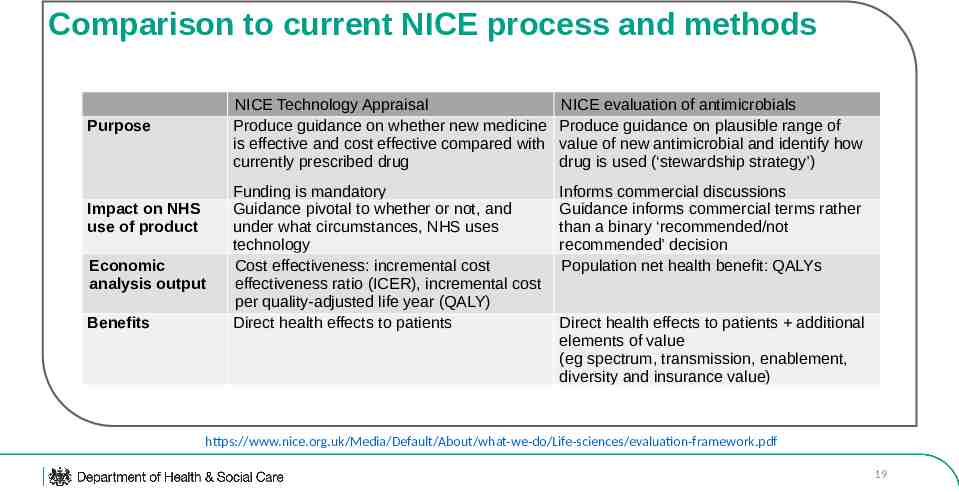
Comparison to current NICE process and methods Purpose Impact on NHS use of product Economic analysis output Benefits NICE Technology Appraisal Produce guidance on whether new medicine is effective and cost effective compared with currently prescribed drug NICE evaluation of antimicrobials Produce guidance on plausible range of value of new antimicrobial and identify how drug is used (‘stewardship strategy’) Funding is mandatory Guidance pivotal to whether or not, and under what circumstances, NHS uses technology Cost effectiveness: incremental cost effectiveness ratio (ICER), incremental cost per quality-adjusted life year (QALY) Direct health effects to patients Informs commercial discussions Guidance informs commercial terms rather than a binary ‘recommended/not recommended’ decision Population net health benefit: QALYs Direct health effects to patients additional elements of value (eg spectrum, transmission, enablement, diversity and insurance value) https://www.nice.org.uk/Media/Default/About/what-we-do/Life-sciences/evaluation-framework.pdf 19
Nice evaluation: how to find out more https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials 20

International collaboration UK represents only a small part of the global market for antimicrobials For our work to have the full effect, we need other countries to test similar models that, together, achieve meaningful incentive for investment Such models would need to be relevant to individual health contexts, but could include shared principles around: Quantitative and/or qualitative assessment of the value of new antimicrobials coming to market Delinking reimbursement to companies from volume of sales Robust stewardship of new and existing antimicrobials Incentivising good manufacturing practice and robust supply 21

International collaboration Sharing project progress and learning is an important part of UK approach. Stakeholder forum Presenting at international fora Meetings with international counterparts G7 Presidency in 2021 provides potential opportunity 22

Questions 23

Thank you! For any further questions or comments, please contact: [email protected]. More information about the project can be accessed at: Models for the evaluation and purchase of antimicrobials Scientific advice Life sciences What we do About NICE 24






