Antibiotics MR. H GEE MD, FRCOG Hon. Assoc. Clinical
80 Slides658.00 KB

Antibiotics MR. H GEE MD, FRCOG Hon. Assoc. Clinical Professor University of Warwick

Objectives By the end of this lecture you should be able to: 1) Classify commonly used antibiotics into six major antibiotic classes of; a) b) c) d) e) f) g) Beta lactams Aminoglycosides Fluoroquinolones Macrolides Tetracyclines Glycopeptides Metronidazole 2) Understand the mechanism of action of each antibiotic class. 3) Understand clinical use of each class of antibiotic 4) Possible major side effects.
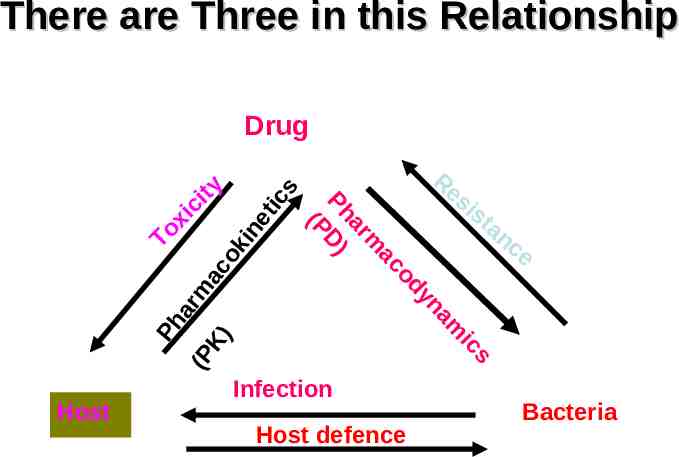
There are Three in this Relationship Host defence s ic Infection m Host Re si st an ce a yn od ac m ar Ph D) (P To Ph xi ar ci (P m ty ac K) ok in et ic s Drug Bacteria

Improving the probability of positive outcomes Window of opportunity – Early recognition and treatment of infection – Selection of appropriate antibiotic (e.g. through in vitro susceptibility determination) – Optimization of DOSE using Pharmacodynamic principles – Use optimized dosing that would allow for the minimization of selecting further resistance
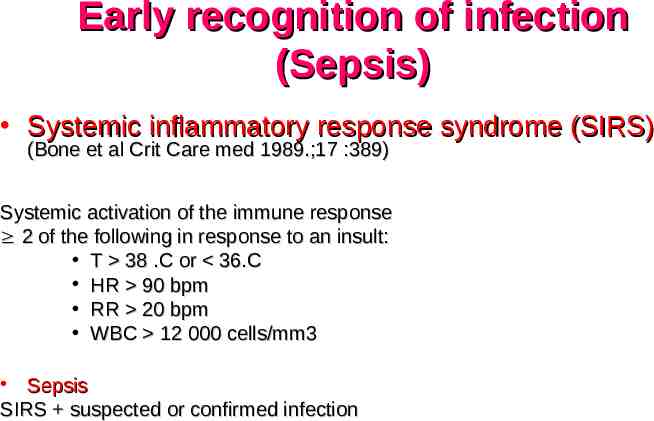
Early recognition of infection (Sepsis) Systemic inflammatory response syndrome (SIRS) (Bone et al Crit Care med 1989.;17 :389) Systemic activation of the immune response 2 of the following in response to an insult: T 38 .C or 36.C HR 90 bpm RR 20 bpm WBC 12 000 cells/mm3 Sepsis SIRS suspected or confirmed infection

Key Message 1 Diagnose sepsis early and give antibiotics promptly to reduce mortality from sepsis

Antibiotics Actions Bactericidal Kills bacteria, reduces bacterial load Bacteriostatic Inhibit growth and reproduction of bacteria All antibiotics require the immune system to work properly Bactericidal appropriate in poor immunity Bacteriostatic require intact immune system
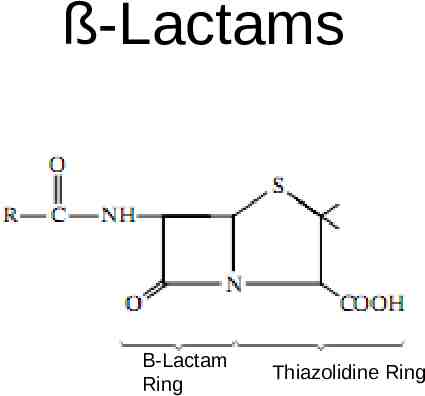
ß-Lactams Β-Lactam Ring Thiazolidine Ring
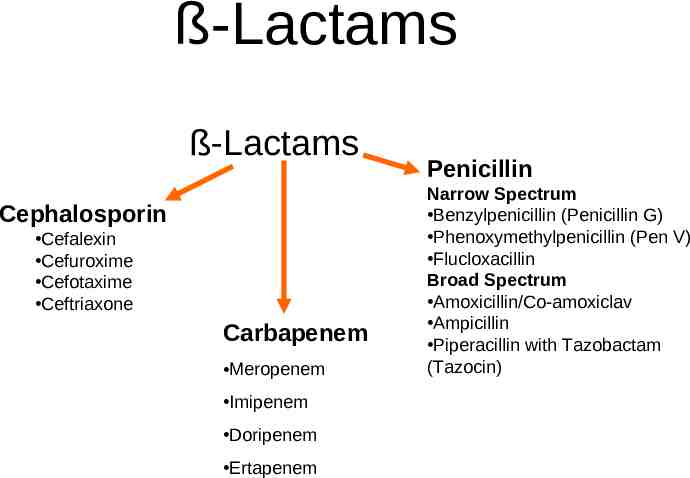
ß-Lactams ß-Lactams Cephalosporin Cefalexin Cefuroxime Cefotaxime Ceftriaxone Carbapenem Meropenem Imipenem Doripenem Ertapenem Penicillin Narrow Spectrum Benzylpenicillin (Penicillin G) Phenoxymethylpenicillin (Pen V) Flucloxacillin Broad Spectrum Amoxicillin/Co-amoxiclav Ampicillin Piperacillin with Tazobactam (Tazocin)

Mechanisms of Action Anti Cell Wall Activity Bactericidal
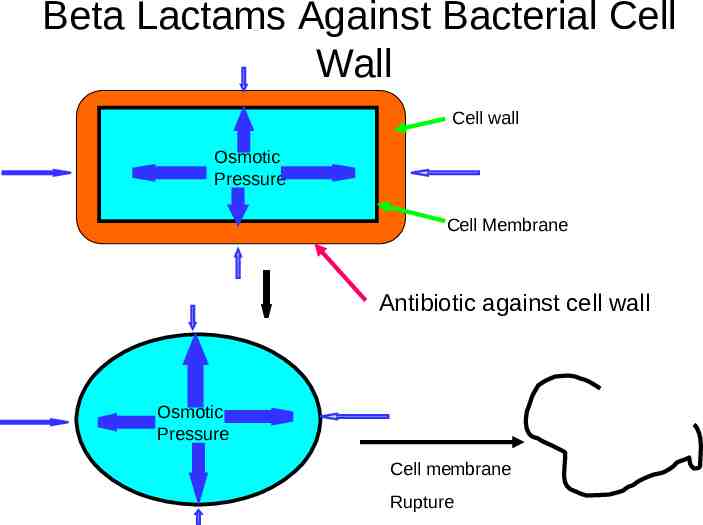
Beta Lactams Against Bacterial Cell Wall Cell wall Osmotic Pressure Cell Membrane Antibiotic against cell wall Osmotic Pressure Cell membrane Rupture

Spectrum of Activity Very wide Gram positive and negative bacteria Anaerobes Spectrum of activity depends on the agent and/or its group

Adverse Effects Penicillin hypersensitivity – 0.4% to 10 % – Mild: rash – Severe: anaphylaxis & death There is cross-reactivity among all Penicillins Penicillins and cephalosporins 5-15%

Resistance to ß-Lactams ß-Lactamase Other mechanisms are of less importance Augmentin

Important Points Beta lactams need frequent dosing for successful therapeutic outcome – Missing doses will lead to treatment failure Beta lactams are the safest antibiotics in renal and hepatic failure – Adjustments to dose may still be required in severe failure

Summary Cell wall antibiotics – Bactericidal Wide spectrum of use – Antibiotics of choice in many infections – Limitations Allergy Resistance due to betalactamase Very safe in most cases – No monitoring required
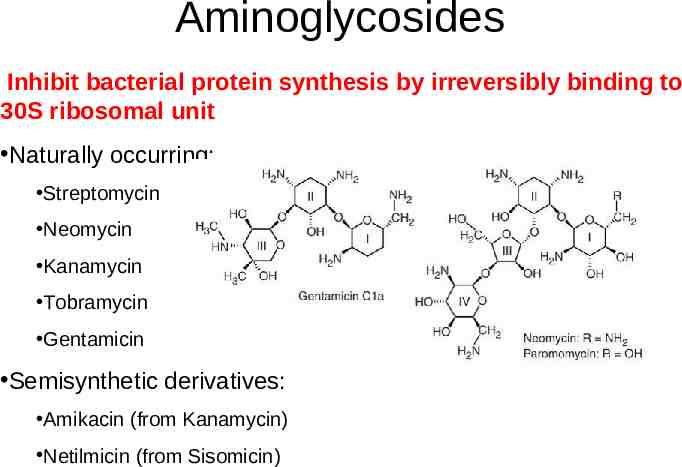
Aminoglycosides Inhibit bacterial protein synthesis by irreversibly binding to 30S ribosomal unit Naturally occurring: Streptomycin Neomycin Kanamycin Tobramycin Gentamicin Semisynthetic derivatives: Amikacin (from Kanamycin) Netilmicin (from Sisomicin)
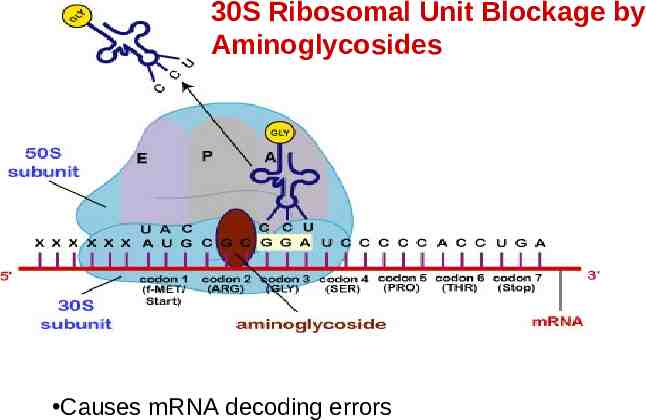
30S Ribosomal Unit Blockage by Aminoglycosides Causes mRNA decoding errors

Spectrum of Activity Gram-Negative Aerobes – Enterobacteriaceae; E. coli, Proteus sp., Enterobacter sp. – Pseudomonas aeruginosa Gram-Positive Aerobes (Usually in combination with ß-lactams) S. aureus and coagulase-negative staphylococci Viridans streptococci Enterococcus sp. (gentamicin)

Adverse Effects Nephrotoxicity – Direct proximal tubular damage - reversible if caught early – Risk factors: High troughs, prolonged duration of therapy, underlying renal dysfunction, concomitant nephrotoxins Ototoxicity – 8th cranial nerve damage – irreversible vestibular and auditory toxicity Vestibular: dizziness, vertigo, ataxia Auditory: tinnitus, decreased hearing – Risk factors: as for nephrotoxicity Neuromuscular paralysis – Can occur after rapid IV infusion especially with; Myasthenia gravis Concurrent use of succinylcholine during anaesthesia

Prevention of Toxicity a) Levels need to be monitored to prevent toxicity due to high serum levels b) To be avoided where risk factors for renal damage exist 1) Dehydration 2) Renal toxic drugs

Mechanisms of Resistance Inactivation by Aminoglycoside modifying enzymes – This is the most important mechanism

Important Points Aminoglycosides should be given as a large single dose for a successful therapeutic outcome – Multiple small doses will lead to treatment failure and likely to lead to renal toxicity Aminoglycosides are toxic drugs and require monitoring – Avoid use in renal failure but safe in liver failure – Avoid concomitant use with other renal toxic drugs – Check renal clearance, frequency according to renal function

Summary Restricted to aerobes Toxic, needs level monitoring Best used in Gram negative bloodstream infections Good for UTIs Limited or no penetration – – – – Lungs Joints and bone CSF Abscesses

Macrolides
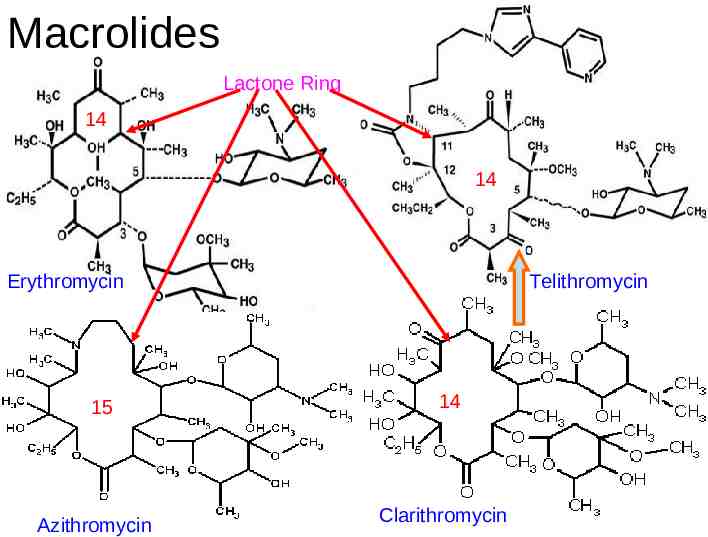
Macrolides Lactone Ring 14 14 Erythromycin 15 Azithromycin Telithromycin 14 Clarithromycin
Mechanism of Action Bacteriostatic- usually Inhibit bacterial RNA-dependent protein synthesis – Bind reversibly to the 23S ribosomal RNA of the 50S ribosomal subunits Block translocation reaction of the polypeptide chain elongation

Spectrum of Activity Gram-Positive Aerobes: – Activity: Clarithromycin Erythromycin Azithromycin MSSA S. pneumoniae Beta haemolytic streptococci and viridans streptococci Gram-Negative Aerobes: – Activity: Azithromycin Clarithromycin Erythromycin H. influenzae, M. catarrhalis, Neisseria sp. NO activity against Enterobacteriaceae Anaerobes: upper airway anaerobes Atypical Bacteria

Mechanisms of Resistance Microlides Altered target sites – Methylation of ribosomes preventing antibiotic binding Cross-resistance occurs between all macrolides

Clinical Use Cellulitis/Skin and soft tissue – Beta haemolytic streptococci – Staphylococcus aureus Intra-cellular organisms – Chlamydia – Gonococcus

Summary Bacteriostatic ALL hepatic elimination Gastrointestinal Sideeffects (up to 33 %) (especially Erythromycin) Nausea Vomiting Diarrhoea Dyspepsia Best used in atypical pneumonia Excellent tissue and cellular penetration – Very useful in susceptible intracellular infections

Fluoroquinolones
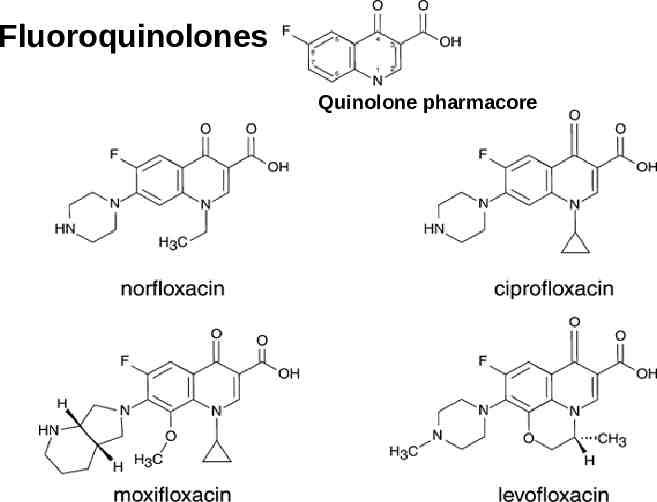
Fluoroquinolones Quinolone pharmacore

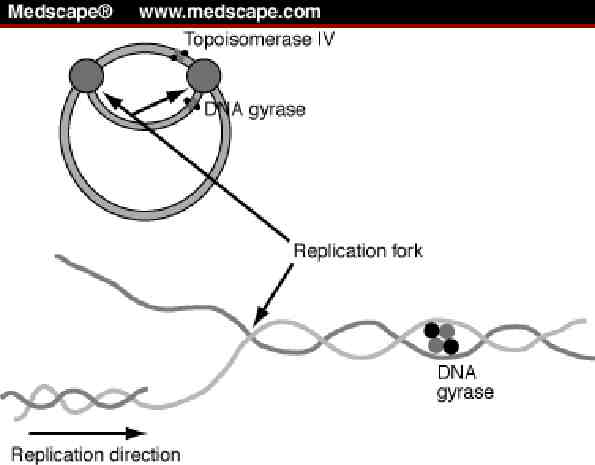
Mechanism of Action Prevent: Relaxation of supercoiled DNA before replication DNA recombination DNA repair

Spectrum of Activity Gram-positive Gram-Negative (Enterobacteriaceae H. influenzae, Neisseria sp. Pseudomonas aeruginosa) – Ciprofloxacin is most active Atypical bacteria: all have excellent activity
Summary Wide range of activity against Gram positive and negative bacteria. Sepsis from Intra-abdominal and Renal Sources – Coliforms (Gram negative bacilli) UTI – E. coli Very good tissue penetration Excellent oral bioavailability High risk for C.difficile
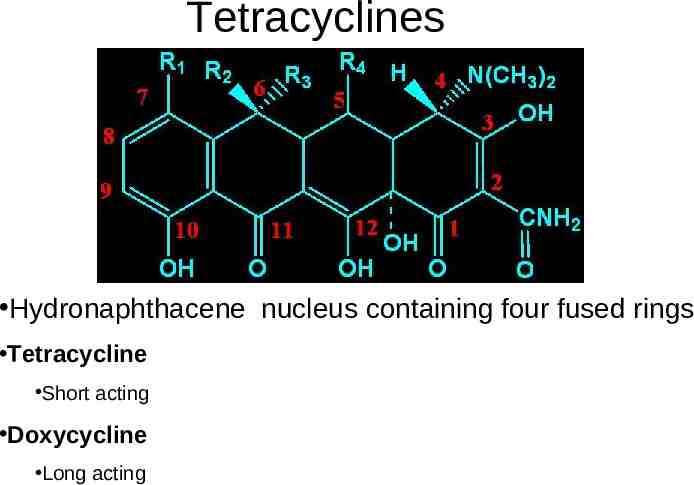
Tetracyclines Hydronaphthacene nucleus containing four fused rings Tetracycline Short acting Doxycycline Long acting

Mechanism of Action Inhibit protein synthesis Bind reversibly to bacterial 30S ribosomal subunits Prevents polypeptide synthesis Bacteriostatic

Spectrum of Activity All have similar activities Gram positives aerobic cocci and rods – Staphylococci – Streptococci Gram negative aerobic bacteria Atypical organisms – – – – Mycoplasmas Chlamydiae Rickettsiae Protozoa
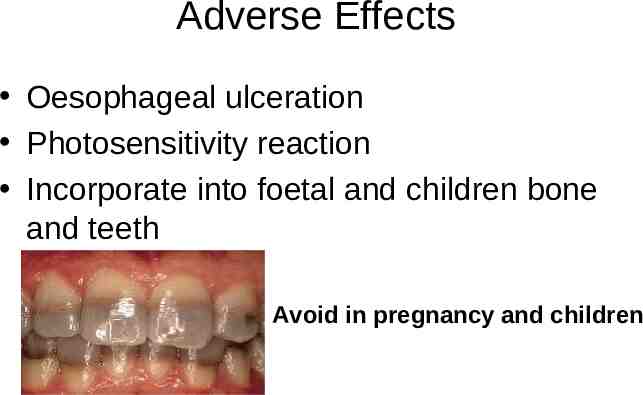
Adverse Effects Oesophageal ulceration Photosensitivity reaction Incorporate into foetal and children bone and teeth Avoid in pregnancy and children

Summary Very good tissue penetration Use usually limited to; – Skin and soft tissue infections – Chlamydia
Glycopeptides Vancomycin Teicoplanin Vancomycin

Mechanism of Action Inhibit peptidoglycan synthesis in the bacterial cell wall Prevents cross linkage of peptidoglycan chains

Summary Large molecule Only active against Gram positive bacteria Second choice in all its uses except; – MRSA – C.difficile
Metronidazole Antibiotic Amoebicide Anti-protozoal – Trichomonas Vaginalis
Mechanisms of Action Molecular reduction – Nitroso intermediates – Sulfamides Melatbolised – Bacterial DNA de-stabilised

Spectrum of Activity & Uses Anaerobes – Bacterial Vaginosis – Pelvic Inflammatory Disease – C. Diff

Bio-Availability Oral Intra-venous – Expensive Rectal – Cheap

Summary Wide spectrum of activity Anaerobes In combination
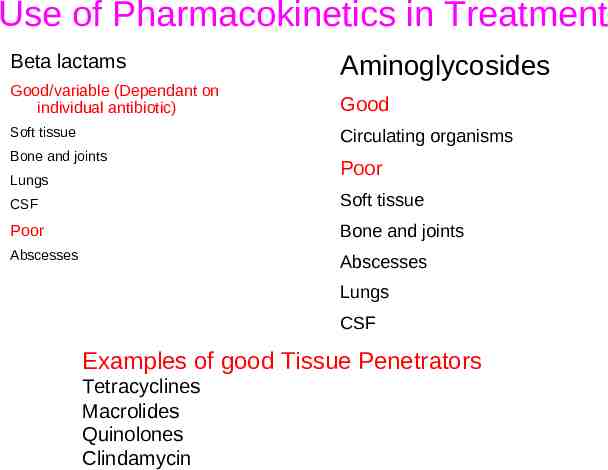
Use of Pharmacokinetics in Treatment Beta lactams Aminoglycosides Good/variable (Dependant on individual antibiotic) Good Soft tissue Circulating organisms Bone and joints Lungs Poor CSF Soft tissue Poor Bone and joints Abscesses Abscesses Lungs CSF Examples of good Tissue Penetrators Tetracyclines Macrolides Quinolones Clindamycin

Key Message 2 When selecting an antibiotic consider the following; – Where is the infection? – Which antibiotics will reach the site of infection Match the two and select your antibiotic

Key Message 3 Always check the impact of an antibiotic on other drugs that a patient is on – Consult BNF or equivalent

PHEW!!! Any Questions?

Chlamydia Trachomatis Obligate, intracellular bacterium Rigid cell wall but NO peptidoglycan layer Cervicitis Slapingitis Pelvic Inflammatory Disease Neonate - mucopurulent conjunctivitis Reiter's syndrome(urethritis, uveitis, arthritis) Lymphogranuloma Venereum

Chlamydia Trachomatis Diagnosis – Giemsa stain Inclusion bodies in epithelial cells Gram stain of no value – ELISA - antigens in exudates or urine – Immunofouresence – PCR – Culture
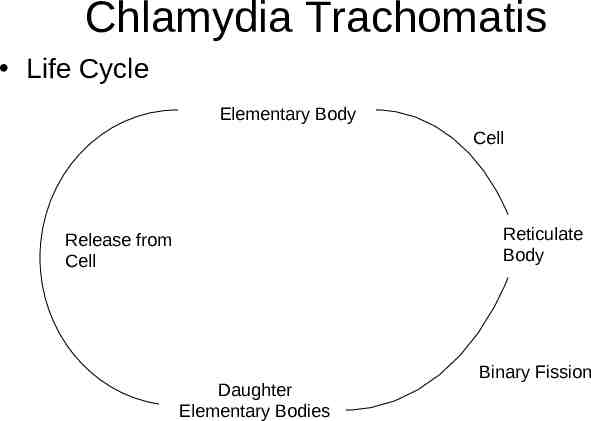
Chlamydia Trachomatis Life Cycle Elementary Body Cell Reticulate Body Release from Cell Daughter Elementary Bodies Binary Fission

Chlamydia Trachomatis Treatment – Tetracyclines (Doxicycline) – Erythromycin – Azythromycin

PK/PD Principles in Antibiotic Prescribing And Prescribing in Organ Failure SAHD May 17, 2013 Peter Gayo Munthali Consultant Microbiologist UHCW Honorary Associate Clinical Professor University of Warwick

Pharmacokinetics - BetaLactams Absorption – PO forms have variable absorption – Food can delay rate and extent of absorption Distribution – Widely to tissues & fluids – CSF penetration: IV – limited unless inflamed meninges Metabolism & Excretion – Primarily renal elimination – Some have a proportion of drug eliminated via the liver – ALL -lactams have short elimination half-lives

Clinical Use - Beta- Lactams Cellulitis/Skin and soft tissues Commonest causes – Beta haemolytic streptococci – Staphylococcus aureus Which Antibiotics? – Benzylpenicillin (Streptococci only) – Flucloxacillin (Staphylococcus aureus and streptococci) Other beta lactams can be used but spectrum too wide

Clinical Use - Beta- Lactams UTI – Commonest cause E. coli – Which antibiotics Cephalexin Co-Amoxiclav – Secondary choice, better non beta lactam alternatives exist » Nitrofurantoin » Trimethoprim

Clinical Use - Beta- Lactams Sepsis from Intra-abdominal and Renal Sources Commonest causes – Coliforms (Gram negative bacilli) Which antibiotics? – Co-Amoxiclav – Tazocin – Meropenem/imipenem/ertapenem (ESBL suspected)

Pharmacokinetics - Aminoglycosides All have similar pharmacologic properties Gastrointestinal absorption: unpredictable but always negligible Distribution – Hydrophilic: widely distributes into body fluids but very poorly into; CSF Vitreous fluid of the eye Biliary tract Prostate Tracheobronchial secretions Adipose tissue Elimination – 85-95% eliminated unchanged via kidney – t1/2 dependent on renal function – In normal renal function t1/2 is 2-3 hours

Clinical Use 1 Aminoglycosides Sepsis from Intra-abdominal and Renal Sources Commonest causes – Coliforms (Gram negative bacilli) Which antibiotics? – Gentamicin/Amikacin (with beta lactam and or metronidazole)

Clinical Use 2 Aminoglycosides UTI Very effective in UTI as 85-95% of the drug is eliminated unchanged via kidney Commonest cause – E. coli – Which antibiotics Gentamicin – Secondary choice, better alternatives exist » Nitrofurantoin » Trimethoprim » Beta lactams
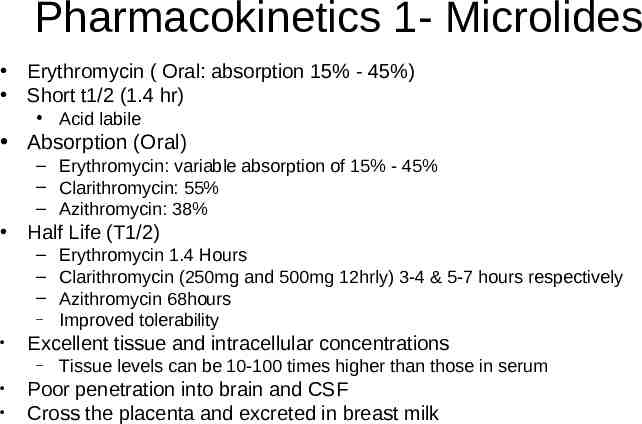
Pharmacokinetics 1- Microlides Erythromycin ( Oral: absorption 15% - 45%) Short t1/2 (1.4 hr) Acid labile Absorption (Oral) – Erythromycin: variable absorption of 15% - 45% – Clarithromycin: 55% – Azithromycin: 38% Half Life (T1/2) – Erythromycin 1.4 Hours – Clarithromycin (250mg and 500mg 12hrly) 3-4 & 5-7 hours respectively – Azithromycin 68hours – Improved tolerability Excellent tissue and intracellular concentrations – Tissue levels can be 10-100 times higher than those in serum Poor penetration into brain and CSF Cross the placenta and excreted in breast milk

Pharmacokinetics 2 - Microlides Metabolism & Elimination – ALL hepatic elimination

Adverse Effects - Microlides Gastrointestinal (up to 33 %) (especially Erythromycin) Nausea Vomiting Diarrhoea Dyspepsia Thrombophlebitis: IV Erythromycin & Azithromycin QTc prolongation, ventricular arrhythmias Other: ototoxicity with high dose erythromycin in renal impairment
Absorption Pharmacokinetics Fuoroquinolones Good bioavailability Oral bioavailability 60-95% Divalent and trivalent cations (Zinc, Iron, Calcium, Aluminum, Magnesium) and antacids reduce GI absorption Distribution Extensive tissue distribution but poor CSF penetration Metabolism and Elimination Combination of renal and hepatic routes

Adverse Effects - Fluoroquinolones Cardiac Prolongation QTc interval Assumed to be class effect Articular Damage Cartilage damage Induced in animals with large doses

Resistance - Fluoroquinolones Altered target sites due to point mutations. The more mutations, the higher the resistance to Fluoroquinolones Most important and most common Altered cell wall permeability Efflux pumps Cross-resistance occurs between fluoroquinolones

Clinical Use 1Fluoroquinolones Sepsis from Intra-abdominal and Renal Sources Commonest causes – Coliforms (Gram negative bacilli) Which antibiotics? – Ciprofloxacin High risk for C.difficile, safer alternatives should be used

Clinical Use 2 Fluoroquinolones UTI – Commonest cause E. coli – Which antibiotics Ciprofloxacin – High risk for C.difficile, safer alternatives should be used

Pharmacokinetics - Tetracyclines Incompletely absorbed from GI, improved by fasting Metabolised by the liver and concentrated in bile (3-5X higher than serum levels) Excretion primarily in the urine except doxycycline ( 60% biliary tract into faeces,40% in urine) Tissue penetration is excellent but poor CSF penetration – Incorporate into foetal and children bone and teeth

Resistance - Tetracyclines Efflux Alteration of ribosomal target site Production of drug modifying enzymes

Clinical Use - Tetracyclines Cellulitis/Skin and soft tissues/ Bone and Joint Infections Commonest causes – Beta haemolytic streptococci – Staphylococcus aureus Which Antibiotics? – Doxycycline

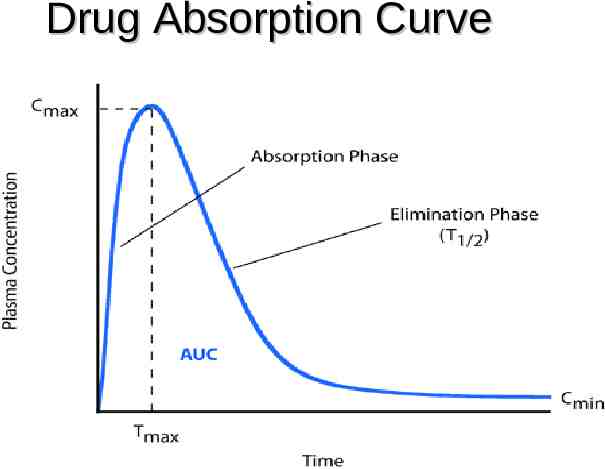
Pharmacodynamics
Drug Absorption Curve
Key Message 4&5 Aminoglycosides are toxic drugs and require monitoring – Avoid use in renal failure but safe in liver failure – Avoid concomitant use with other renal toxic drugs – Check renal clearance, frequency according to renal function Beta lactams are the safest antibiotics in renal and hepatic failure – Adjustments to dose may still be required in severe failure







