ACCELERATED STABILITY TESTING
39 Slides445.81 KB

ACCELERATED STABILITY TESTING

Stability: Stability of pharmaceutical product may be defined as the capability of a particular formulation in a specific container/closure system to remain within its physical, chemical, microbiological, therapeutic and toxicological specification.

According to USP Types of Stability Type condition to be maintained 1) Chemical Chemical integrity & labeled potency 2) physical Appearance & palatability , uniformity 3) microbiological sterile 4) therapeutic Drug should remain potent 5) toxic Should not be toxic

Stability testing & problems Pharmaceutical products often may exhibit physical or chemical reaction that may end in instability. This degradation may lead to 1)Reduced activity of preparation 2)Formation of toxic products 3)Inelegant product Stability testing is necessary to ensure the degradation has not exceed an acceptable level assuring 1)Safety of the patient 2)Activity of the product

Degradation reactions: Hydrolysis Oxidation-reduction Racemisation Decarboxylation Ring cleavage Photolysis Isomerisation

Hydrolysis: Many pharmaceutical preparations contain ester, amide groups Ester hydrolysis: The hydrolysis of an ester in to mixture of acid &alcohol essentially involves the rupture of a covalent linkage between carbon atom &oxygen atom Ester H (OR) OH - Acid Alcohol Drugs under go hydrolysis –procaine, atropine, asprin

Amide hydrolysis: Pharmaceutical compound containing amide under go hydrolysis it gives acid &amine Amide h2o acid amine Drugs under go amide hydrolysis – niacinamide, phenethicillin, chloramphenicol, barbiturates

Protection against hydrolysis: By avoiding contact with water vapour control of atmospheric humidity during preparation & packing Adjusting pH to an optimum level Hydrolytic reactions generally minimized by partial (or) full replacement of water with lower dielectric constant sol such as glycol, glucose, mannitol By modification of chemical structure by increasing the length of branching the alkyl groups, hydrolysis of ester may be decreased by owing to steric hindrance.

Oxidation-reduction: Oxidation involves addition of oxygen, removal of hydrogen Fe Fe 1e- RH Oxidative Drugs R0 (H) free radical degradation influenced by light and heat which under go oxidative decomposition are ergometrine, heparin, tetracyclines, amikacine , morphine, neomycine, norepinephrine, paraldehyde, reserpine, terpenes, tubacurarine, riboflavin, physostigmine, vitaminD, K, C

Initiation RH R (H) PROPAGATION R . O2 RO2 RO2 . RH ROOH R . HYDROPEROXIDE DECOMPOSITION ROOH RO . . OH TERMINATION RO2 . X INACTIVE PRODUCT RO2 RO2 INACTIVE PRODUCT

Protection against oxidation: Effective ness of antioxidants can be increased by use of synergists such as chelating agents, also depend on conc used, ph, packing Oil soluble anti oxidants(hydroquinone, ascorbyl palmitate) prevent free radical chain process To prevent oxidation replacing the air with inert gas nitrogen in the ampoules.

photolysis Degradation of product due to absorption of radiation energy in the form of light The radiations absorbed from the ultra violet & violet portions of the light spectrum are more active in initiating chemical reactions. Free radicals are produced due to photolysis reaction that lead to degradation , photo chemical reactions are accompanied by thermal reaction Photo derivative reactions follow second order, first order, zero order. Photo degradation of chlorpromazine hydrochloride 253.5mµ , the uv irradiation of chlorpromazine cause degradation to proceed through a semi quinone free radical Drugs which under go photo degradation are nifedipine, fursemide, Chloropromazine Photochemical reactions can be reduced by storing product in darkness, amber coloured bottles, packing in cartons.

Ring alteration: A hydrolytic reaction can proceed as result of cleavage with subsequent attack by hydrogen or hydroxyl ion Drugs under go hydrolysis due to ring cleavage are hydrochlorothiazide, pilocarpine , reserpine

Racemization: Stability of pharmaceutical formulation, the biological effect of the dextro form can be considerably less than levo form. For example levo-adrenaline is 15 to 20 times more active than dextro –adrenaline. Sol of levo-adrenaline form a racemic mix of equal parts of levo & dextro-adrenaline with a pharmacological activity just over half that of pure compound Racemization similar to hydrolytic reaction The racemization of a compound appears to depend on the asymmetric carbon atom

Accelerated stability testing: pharmaceutical formulations are stored under normal conditions, their instabilities are detectable after only long storage & such methods are time consuming & uneconomical , to overcome these problems preparations are tested under stress conditions , which will accelerate decomposition at a faster rate ,by this instabilities can be detected OBJECTIVES: To select best formulation To predict shelf life Used in quality control

Shelf life: t90 : Time required to reduce the concentration to 90% of its initial concentration. t90 0.105/K stability of formulation can be determined by shelf life.

Calculation of shelf life:

Arrhenius equation Temperature is probably the most common acceleration factor used for chemicals, pharmaceuticals, and biological products since its relationship with the degradation rate is well characterized by the Arrhenius equation. According to Arrhenius, for every 10 0c rise in temperature the speed of reaction increases about 2-3 times. K Ae –Ea/RT A is Arrhenious factor Ea is Energy of activation R is Gas constant Arrhenius factor is frequency of molecular collisions occurring between the molecules. Log K Log A-Ea/2.303RT

ACTIVATION ENERGY: It is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction. The activation energy of a reaction is usually denoted by Ea

Estimation of activation energy: A graph can be drawn by taking log k on y-axis and reciprocal temperature (1/T) on x-axis. A straight line is obtained, the slope of the line is negative and the magnitude is Ea /2.303 R. The intercept corresponds to log A. All the constants in the Arrhenius equation can be obtained from the graph.

Prediction of shelf life The stability of any active component in a pharmaceutical preparation can be evaluated by determining some property of degradation ( i.e colour disappearance) , temp dependency of degradation can be obtained with help of arrhenius eq

Various steps involved in prediction of shelf life

Preparation made to 5portions, stored at diff temp 40 ,50 ,60 ,70 ,80 Samples are with drawn at diff intervals Order of reaction is determined by plot of time &conc , by Arrhenius eq from graph k value at 25 can be known, Used to estimate time during which drug remain potent Appropriate calculation is carried out to find out the amount of drug to be added in excess(overages)

Example Absorbance at time t (At ) 0.225 absorbance initially ( A0 ) 0.470 Rate constant ( K) 2.09 X 10 -5 At A0-Kt Kt -At A0 t -At A0/K Substituting the values t (-0.225 0.470)/2.09 x 10 -5 t 11722 hrs (i.e. 1 year 4 months)
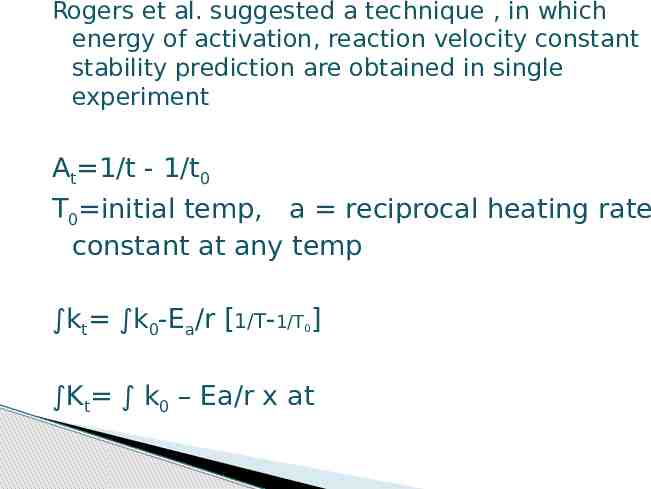
Rogers et al. suggested a technique , in which energy of activation, reaction velocity constant stability prediction are obtained in single experiment At 1/t - 1/t0 T0 initial temp, a reciprocal heating rate constant at any temp kt k0-Ea/r [1/T-1/T ] 0 Kt k0 – Ea/r x at

Distribution of world nations into different zones: Zones Condition Temperature Relative humidity Zone 1 Temperature 200c 42 Zone 2 Sub tropical 220c 52 Zone 3 hot/dry 27.90c 35 Zone 4 Hot/humid 27.40c 76

Different zones: zones Zone 1 countries Great Britain, North Europe, Russia , Canada Zone 2 US, Japan, South Europe Zone 3 Iran, Iraq, Sudan Zone 4 Brazil, Ghana, Philippines Indonesia,

Storage conditions STABILITY STUDY STORAGE TESTING CONDITIONS FREQUENCY (MONTHS) Accelerated 40 2ºC & 75 5% 0,1,2,3 & 6 RH Intermediate 30 2ºC & 65 5% 0,3,6,9,12,18,24 & 36 RH Long term 25 2ºC & 60 5% 0,3,6,9,12,18,22,24,2 RH 6,36,48 & 60

As per ICH, WHO, FDA Storage condition for accelerated testing according to ICH and WHO is 400c 20c 75%RH 5% If the product is unstable in above conditions intermediate conditions are used 300c 20c 65% RH 5% FDA prescribes 0,2,4 and 6 months. WHO prescribes 0, 1,2,3,4 and 6 months. ICH prescribes 3 months in 1 year and frequency of 6 months in 2 year and then annually

Accelerated stability: test for photochemical It can be done by inducing rapid decomposition by using artificial light source. The intensity of light is proportional to photo degradation of formulation. TO OVER COME THE PROBLEM : To overcome photochemical degradation, the formulation must be packed in amber coloured containers and extra protection from light is provided by placing the container in carton box.

Accelerated test for moisture absorption: In these the products are placed in small cabinets containing different saturated salt solution which maintain high relative humidity and controlled temperature. The formulations are kept in packed and unpacked forms and are checked for their physical and chemical stability. It indicates the product is susceptible to moisture or not.

Accelerated test for emulsions For emulsion we cannot perform accelerated test by increasing temperature because at higher temperature, the emulsion will break. So we perform centrifugation instead of increasing temperature. By centrifugation we accelerate the rate of creaming. Ultra centrifuges used with 60,000rpm. Rate of creaming is proportional to speed of centrifuge. The emulsion is subjected to different centrifugal speeds and separation of phases is observed at different time periods. Bad emulsion separates oil instantly. Good emulsion does not exhibit detectable separation of oil phase until certain time period.

Accelerated test for suspension Cake formation is accelerated by centrifugation. High speed centrifugation is hence not preferred, low speed centrifugation is used to study physical stability. A freeze-Thaw cycling technique is one of the stress testing. Freeze-thaw methods: A freeze thaw cycling technique is one of the stress testing's. This cycling treatment promotes particle size, particle size distribution and crystal habit.

LIMITATIONS OF ACCELERATED STABILITY TESTING Valid only when the break down depends on temperature. The energy of activation obtained in the study should be between 10 to 30 kcal/mole. It is not useful when degradation is due to: Microbial contamination Photochemical reactions Diffusion Excessive agitation When the product looses its physical integrity at higher temperatures. When the order changes at elevated temperatures.

Standard Title and reference ICH Q1A(R2) Stability Testing of New Drug Substances and Products (the parent guideline) ICH Q1B Photostability Testing of New Drug Substances and Products ICH Q C Stability testing of new dosage forms ICH D Bracketing and matrixing designs ICH Q E Evaluation of stability data ICH Q F Stability data package for registration applications in climatic zone I and IV

Good manufacturing practices & expiration dating Good manufacturing practices (GMP) required for drug stability(section 211.166) expiration date (section 211.137), & FDA guidelines for stability studies (section 98) contain significant & specific information related to conducting stability studies and assigning expiration dates Each product expiration date related to the specific storage condition stated on the label Stability include ,no & size of containers for sample, testing the product in market , adequate no of batches Expiration date derived from stability studies Drug product packed in diff pack ,it retain expiration date , if it the repackaged can assure the FDA that the repacked container is as good as original package product specifications can be maintained through out the period

REFERENCES: Text book of Physical Pharmacy by Manavalan Patrick J. Sinko, Martin’s Physical Pharmacy and Pharmaceutical sciences Theory and practice of industrial pharmacyLachman Drug stability by Cartensen C.V.S Subrahmanyam text book of Physical Pharmacy Pharmaceutical dosage forms by aulton International stability testing by david j.mazzo Hand book of stability testing in pharmaceutical development(regulations, methodologies &best practices) edited by kim huynh

Acknowledgement : I take this opportunity to express my sincere gratitude to our esteemed teachers Prof. A. seetha devi madam(HOD of pharmaceutics department), prof.D.varun sir, &g.y.srawan sir, A.Ankka rao sir ,v.vasu naik sir, p.dinesh sir, entire pharmaceutics department who have suggested me with their valuable contributions suggestions and constructive criticisms in the most appropriate way.







