Guidance in PROTOCOL SUBMISSION to Barry University IRB May 1, 2022
16 Slides118.75 KB

Guidance in PROTOCOL SUBMISSION to Barry University IRB May 1, 2022

Objectives of this Guidance Explanation of Submission Types Full board Exempt Expedited How to Prepare a Submission Tips for Preparing a Successful Submission Clearly explain on your Protocol what you will ask of participants Consider using boilerplate language Use the correct Barry Logo on your documents Take the correct Ethics Training What Happens Next?

Submission types Full Board review: When research involves more than minimal risk or otherwise requires review by the Full Board, it is scheduled to be discussed at the next IRB meeting. It is recommended that you attend the meeting in which your research is being discussed (schedule a remote connection with the IRB point of contact, Anoush McNamee at 305-899-3020 or [email protected]) Submissions must be made before the first Friday of the month to be considered for Full Board review at that month’s meeting. Exempt review: Most research meets criteria for being exempt from Full Board review. Exempt Reviews must meet federal guidelines for exemption. Exemption categories are indicated on the form that researchers complete for the IRB review (the “Protocol form”) and detailed information about exemption categories is in the Appendices of that form. Protocols determined to be exempt have no expiration date and require no annual report. Expedited review: Expedited Review is rare and should only be requested when time constraints prevent you from waiting to begin collecting data. If you request an expedited review, you must justify why you cannot wait to go through the normal review process.
How to Prepare a Submission Your first step is to create an account on IRBNet so you can submit research “packages” for review. There is a Guidance document on the IRB Website that gives detailed instructions for how to create an account. Once you have an account, you are ready to take the required ethics trainings (see Slide 13 here, and the Guidance document on the IRB Website for detailed instructions). Your certificates of completion should be uploaded to your User File so they can be easily attached to any of your packages. After completing the required training, complete a “Protocol Form” to summarize your study for IRB review. All submission types use the same Protocol Form; the Barry University Institutional Review Board Protocol Form (the current version is the one approved on 4/27/2022). Download the form from “Forms & Templates” (a menu option on IRBNet). Directions for completing the Protocol form are embedded within the form. If you need further clarification, detailed instructions are provided with the Video Instructions for IRB Users link located on the IRB Webpage. When completed, the Protocol form should be uploaded to your IRBNet package. All documents related to the study should also be uploaded as separate items (i.e., Participant recruitment materials, Consent Form, Interview questions, Surveys) Lastly, all members of the research team (those who will have contact with participants and/or identifiable participant data) must upload their ethics training certificates to the package. The package must be electronically signed.

Tip: Clearly explain on the Protocol What You Will Ask of Participants The IRB is responsible for assuring that researchers at Barry treat participants ethically. In order to determine if the intended research is ethical, the IRB member reviewing your Protocol must clearly understand exactly what your participants will experience. The “Method” section of the Protocol is where you specify, step by step, what will happen to your participants. Include the total time commitment required of participants and be sure you report the time consistently throughout your documents. For example, you might itemize each step like so: Participants will sign a Consent form After consenting, they will complete a Demographic Questionnaire created for the study They will then view 1 of 4 pictures of a psychotherapist’s office and asked to rate the office on a Likert scale with anchors “cold” to “warm” Finally, participants will be asked to imagine the therapist who works in the office they saw, and complete the Psychotherapist Qualities Inventory The expected time commitment is no more than15 minutes

Tip: Consider Using Boilerplate Language Note that this is not required language, but rather examples of language that meet IRB standards. Using boilerplate language does not guarantee approval as is. The five (5) slides that follow provide boilerplate language for: Benefits statement Risk statement Number of Participants statement Anonymity/Confidentiality statement Using Data in Future Studies statement

Boilerplate Language: Benefits Statement In the Protocol There are no direct benefits to participants. There are no direct benefits to participants. However, the research will inform our understanding In the Consent document (writing to potential participants) There are no direct benefits to you for participating in the study. Although there may be no direct benefit to you, the research will help us learn more about

Boilerplate Language: Risk Statement For studies with minimal risk: There are no known risks to participating in this study. Risks to participating in this study are no greater than those encountered in everyday life. For studies with risk, you must describe the risks and address what you will do should there be a negative outcome to participants, such as providing referral information regarding available services to mitigate distress. Risks to participating in this study include Should you feel the need for support following your participation in this study, please call xxx hotline number at

Boilerplate Language: Number of Participants Statement When assessing the costs versus the benefits of a study, the IRB reviewer takes into consideration how may people will be exposed to the study (in part or whole). Therefore, the maximum number of participants that will possibly be recruited is the information needed. Be sure you provide a number that is more than what you need for data analysis because you are likely to have attrition and/or unusable data. The number of interest to the IRB is the number of people who may be exposed to your study, not the number you need for data analysis. The maximum number of participants that will be recruited is 150. This allows for an adequate sample while also accounting for attrition and incomplete or otherwise unusable data.

Boilerplate Language: Anonymity/Confidentiality Statement Do not cut and past this paragraph. Edit the paragraph as necessary so it fits with your study. For example, if you are not using focus groups or audio recordings, you should remove reference to those items. All study data (i.e., observation, interview, focus group, implementation, and outcome data) will be kept confidential. No participant, site, or institution names will be used in reports of the findings. Participants’ names (including consent forms) will be stored separately from the data. Audio recordings will be captured and stored digitally on password protected devices and transcribed by researchers. In the event that external transcription is sought, nonresearcher transcriptionists will sign confidentiality agreements. Electronic data (including transcripts) will be stored on password protected computers. Hard copy data will be locked in a researcher’s office. All data will be kept indefinitely, for at least five years following completion of the study. If it becomes necessary to destroy the data at a later time, electronic data will be destroyed by double deleting from the hard drive and Recycle Bin. Hard copy data will be shredded.

Boilerplate Language: Future Use of Deidentified Data A statement about whether data will be used in the future is required on Consent documents. These data—with identifiers removed--may also be used in future research or distributed to another investigator without additional informed consent. These data will not be used or distributed for future research studies.
Tip: Use the Correct Barry Logo on Your Documents Any research document that is created for your study must contain a Barry logo. For example: Recruitment flyer Consent form Gatekeeper letter To avoid the improper use of available logos – such as using the President’s Seal – the IRB would like the logo shown here to be the one used by researchers.
Tip: Take the Correct Ethics Training Detailed instructions for how to find and take the required ethics training modules can be found on the IRB Website in Practical Guidance in CITI CERTIFICATION for Barry University IRB. In summary, all people working with participants and/or identifiable participant data must complete two (2) training modules. The modules are identical aside from the examples used so either set of modules is appropriate, but you may want to take the modules that best fit their discipline: The modules are: 1. Social and Behavioral Research-Basic Refresher course AND the Social and Behavioral Responsible Conduct of Research course OR 2. Biomedical Research-Basic Refresher course AND the Biomedical Responsible Conduct of Research course
What Happens Next: A Full Board Review Either you or an IRB Step reviewer notes that the study requires a 1 Full Board review The IRB Chair Stepverifies that the study requires a Full 2 Board review The study is Step scheduled for review at the next IRB 3 meeting
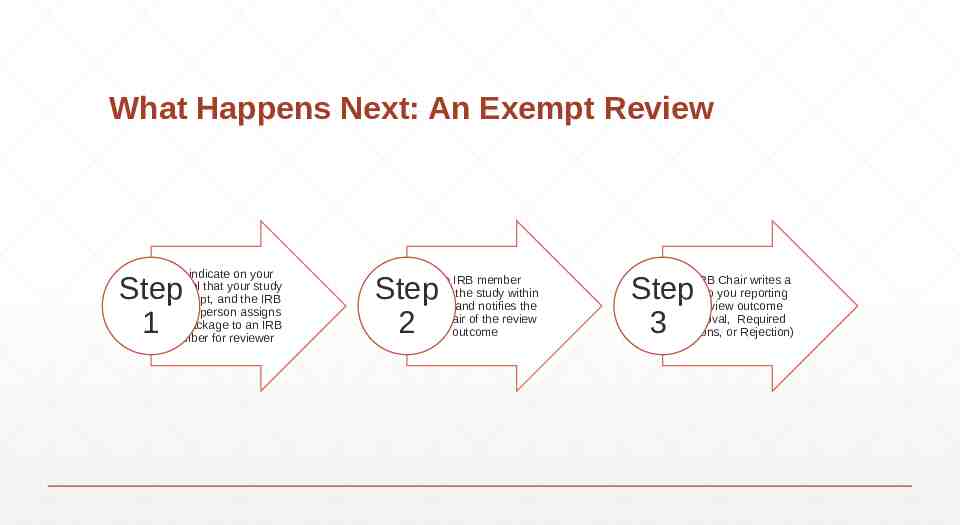
What Happens Next: An Exempt Review You indicate on your Protocol that your study is Exempt, and the IRB contact person assigns your package to an IRB member for reviewer Step 1 Step 2 The IRB member reviews the study within 1 week and notifies the IRB Chair of the review outcome Step 3 The IRB Chair writes a letter to you reporting the review outcome (Approval, Required Revisions, or Rejection)
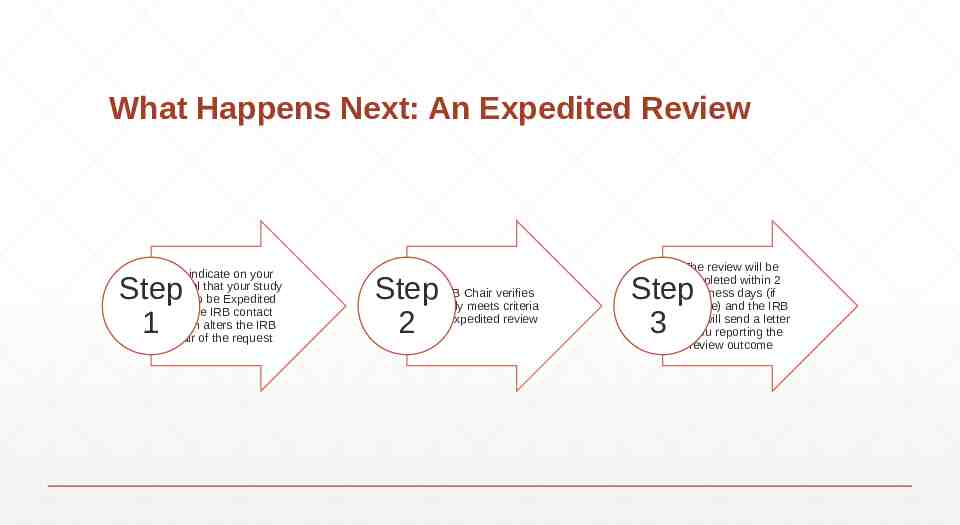
What Happens Next: An Expedited Review You indicate on your Protocol that your study need to be Expedited and the IRB contact person alters the IRB Chair of the request Step 1 Step 2 The IRB Chair verifies that study meets criteria for an Expedited review The review will be completed within 2 business days (if possible) and the IRB Chair will send a letter to you reporting the review outcome Step 3






